Team:Chiba/Calendar-Home/25 August 2008
From 2008.igem.org
(Difference between revisions)
(→Team:Communication) |
(→Team:Output) |
||
| Line 168: | Line 168: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA tamplate</td> | + | <td>DNA tamplate(μL)</td> |
<td>1</td><td>1</td><td>1</td><td>1</td><td>1</td> | <td>1</td><td>1</td><td>1</td><td>1</td><td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>FW primer</td> | + | <td>FW primer(μL)</td> |
<td>5(Venus_YFP_fwd)</td><td>5(LacZ_fwd)</td><td>5(LacZ_fwd)</td><td>5(rLUC_fwd)</td><td>5(fLuc_fwd)</td> | <td>5(Venus_YFP_fwd)</td><td>5(LacZ_fwd)</td><td>5(LacZ_fwd)</td><td>5(rLUC_fwd)</td><td>5(fLuc_fwd)</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>VR primer</td> | + | <td>VR primer(μL)</td> |
<td>5(VR)</td><td>5(LacZ_rev)</td><td>5(LacZ_rev)</td><td>5(VR)</td><td>5(VR)</td> | <td>5(VR)</td><td>5(LacZ_rev)</td><td>5(LacZ_rev)</td><td>5(VR)</td><td>5(VR)</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>dNTPmix</td> | + | <td>dNTPmix(μL)</td> |
<td>10</td><td>10</td><td>10</td><td>10</td><td>10</td> | <td>10</td><td>10</td><td>10</td><td>10</td><td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Thermo pol buffer</td> | + | <td>Thermo pol buffer(μL)</td> |
<td>10</td><td>10</td><td>10</td><td>10</td><td>10</td> | <td>10</td><td>10</td><td>10</td><td>10</td><td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA pol(VENT)</td> | + | <td>DNA pol(VENT)(μL)</td> |
<td>1</td><td>1</td><td>1</td><td>1</td><td>1</td> | <td>1</td><td>1</td><td>1</td><td>1</td><td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>68</td><td>68</td><td>68</td><td>68</td><td>68</td> | <td>68</td><td>68</td><td>68</td><td>68</td><td>68</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
| - | <td> | + | <td>100</td><td>100</td><td>100</td><td>100</td><td>100</td> |
</tr> | </tr> | ||
</table> | </table> | ||
| Line 210: | Line 210: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA tamplate</td> | + | <td>DNA tamplate(μL)</td> |
<td>1</td><td>1</td><td>1</td><td>1</td><td>1</td> | <td>1</td><td>1</td><td>1</td><td>1</td><td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>loading Dye</td> | + | <td>loading Dye(μL)</td> |
<td>1</td><td>1</td><td>1</td><td>1</td><td>1</td> | <td>1</td><td>1</td><td>1</td><td>1</td><td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>4</td><td>4</td><td>4</td><td>4</td><td>4</td> | <td>4</td><td>4</td><td>4</td><td>4</td><td>4</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
| - | <td> | + | <td>6</td><td>6</td><td>6</td><td>6</td><td>6</td> |
</tr> | </tr> | ||
</table> | </table> | ||
| Line 246: | Line 246: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA tamplate</td> | + | <td>DNA tamplate(μL)</td> |
<td>1</td> | <td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>FW primer(Venus)</td> | + | <td>FW primer(Venus)(μL)</td> |
<td>5</td> | <td>5</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>VR primer</td> | + | <td>VR primer(μL)</td> |
<td>5</td> | <td>5</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>dNTPmix</td> | + | <td>dNTPmix(μL)</td> |
<td>10</td> | <td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Thermo pol buffer</td> | + | <td>Thermo pol buffer(μL)</td> |
<td>10</td> | <td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA pol(VENT)</td> | + | <td>DNA pol(VENT)(μL)</td> |
<td>1</td> | <td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>68</td> | <td>68</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
| - | <td> | + | <td>100</td> |
</tr> | </tr> | ||
</table> | </table> | ||
Latest revision as of 06:15, 30 October 2008
24 August 2008 <|> 26 August 2008
Contents |
Laboratory work
Team:Input
The following glycerol Stock was made.
BBa_J22136(insert check OK)
Picked the colony and incubate with 2mL of LB-Ampicillin Medium for 12 hours at 37 degrees.
Took 180μl from culture,and mixed with 880μlLB-Amp Medium.(OD=0.485)
| Liquid culture(OD=0.495) | 0.67μl |
| 60%Glycerol | 0.33μl |
| 20%Glycerol Stock | 1.00ml |
Team:Communication
- (24/8)--->Digestion
- Double Digestion:BBa_J04500(2007)
- Double Digesiton:BBa_R0010(2007)
- Single Digestion:BBa_R0010(2007)
- BBa_R0010(2007)
| Sample No. | 1 | 2 | 3 | 4 |
| Sample DNA(μL) | 5 | 5 | 1 | 1 |
| EcoRI(μL) | 0.5 | 0.5 | - | - |
| SpeI(μL) | 0.5 | 0.5 | - | - |
| Buffer 1 | 1 | 1 | - | - |
| Buffer 3(μL) | - | - | 1 | - |
| BSA(μL) | 1 | 1 | - | - |
| dH2O(μL) | 2 | 2 | 7.5 | - |
| TOTAL(μL) | 10 | 10 | 10 | 1 |
- --->Gel Check
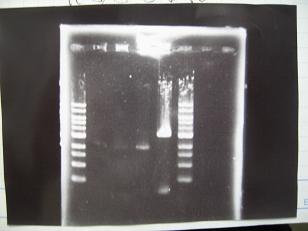
|
|
--->Digestion
(Double Digestion)
- BBa_C0178
- BBa_C0170
- BBa_J04500(2007)
| Sample No. | 1 | 2 | 3 |
| Sample DNA(μL) | 33ng/μl×60μl | 33ng/μl×60μl | 100ng/μl×20μl |
| PstI(μL) | 2 | 2 | 2 |
| XbaI(μL) | 2 | 2 | - |
| SpeI(μL) | - | - | 2 |
| Buffer 2(μL) | - | - | 5 |
| Buffer 3(μL) | 10 | 10 | - |
| BSA(μL) | 10 | 10 | 5 |
| dH2O(μL) | 16 | 16 | 16 |
| TOTAL(μL) | 100 | 100 | 52 |
Team:Output
- BBa_E2030(1)
- BBa_J33202(2)
- BL21(3)
- BBa_J52008(4)
- BBa_I712019(5)
| No. | 1 | 2 | 3 | 4 | 5 |
| DNA tamplate(μL) | 1 | 1 | 1 | 1 | 1 |
| FW primer(μL) | 5(Venus_YFP_fwd) | 5(LacZ_fwd) | 5(LacZ_fwd) | 5(rLUC_fwd) | 5(fLuc_fwd) |
| VR primer(μL) | 5(VR) | 5(LacZ_rev) | 5(LacZ_rev) | 5(VR) | 5(VR) |
| dNTPmix(μL) | 10 | 10 | 10 | 10 | 10 |
| Thermo pol buffer(μL) | 10 | 10 | 10 | 10 | 10 |
| DNA pol(VENT)(μL) | 1 | 1 | 1 | 1 | 1 |
| dH2O(μL) | 68 | 68 | 68 | 68 | 68 |
| TOTAL(μL) | 100 | 100 | 100 | 100 | 100 |
-->Gel Check
| No. | 1 | 2 | 3 | 4 | 5 |
| DNA tamplate(μL) | 1 | 1 | 1 | 1 | 1 |
| loading Dye(μL) | 1 | 1 | 1 | 1 | 1 |
| dH2O(μL) | 4 | 4 | 4 | 4 | 4 |
| TOTAL(μL) | 6 | 6 | 6 | 6 | 6 |
- BBa_E2030
- PUC19
- pGFPUV
- pGEX Venus YFP
- Membren Venus YFP
- BBa_J63001(1)
| No. | 1 |
| DNA tamplate(μL) | 1 |
| FW primer(Venus)(μL) | 5 |
| VR primer(μL) | 5 |
| dNTPmix(μL) | 10 |
| Thermo pol buffer(μL) | 10 |
| DNA pol(VENT)(μL) | 1 |
| dH2O(μL) | 68 |
| TOTAL(μL) | 100 |
 "
"