Team:Chiba/Experiments:LuxR mutant
From 2008.igem.org
(→Design) |
|||
| (40 intermediate revisions not shown) | |||
| Line 13: | Line 13: | ||
| - | ==LuxR mutant ( | + | ==LuxR mutant (Ongoing)== |
===Design=== | ===Design=== | ||
| - | + | Probably, the most straightforward approach to make variations in delay time is to create a number of LuxR mutants with different sensitivity. We thought the higher the sensitivity is, the shorter the delay time would be. The lower the sensitivity is, the slower the switch response should be. | |
| - | + | ||
| - | ::: | + | At least, two papers on LuxR mutants [[Team:Chiba/Experiments:LuxR mutant#Reference|<sup>(1),(2)</sup>]] are available. Among the many candidates, we chose the following three mutations; |
| - | + | #Ile45Phe: This mutation make LuxR 10x more sensitive to Lux-type AHL[[Team:Chiba/Experiments:LuxR mutant#Reference|<sup>(1)</sup>]] | |
| - | + | #Leu42Ala: Sensitivity (to AHL) becomes 1/15 of the wildtype LuxR[[Team:Chiba/Experiments:LuxR mutant#Reference|<sup>(2)</sup>]] | |
| - | [[ | + | #Leu42Ser: Sensitivity (to AHL) becomes 1/1,000 of the wildtype LuxR[[Team:Chiba/Experiments:LuxR mutant#Reference|<sup>(2)</sup>]] |
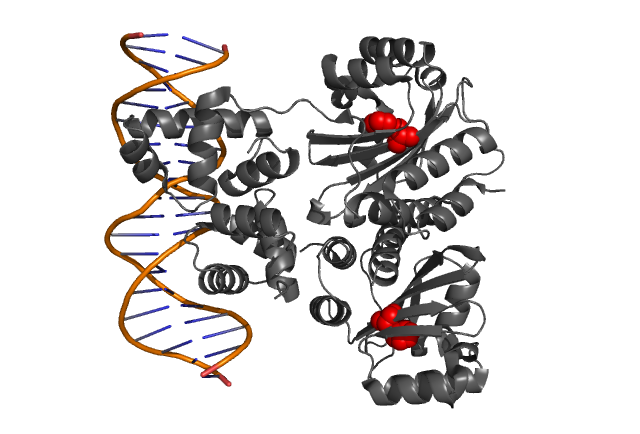
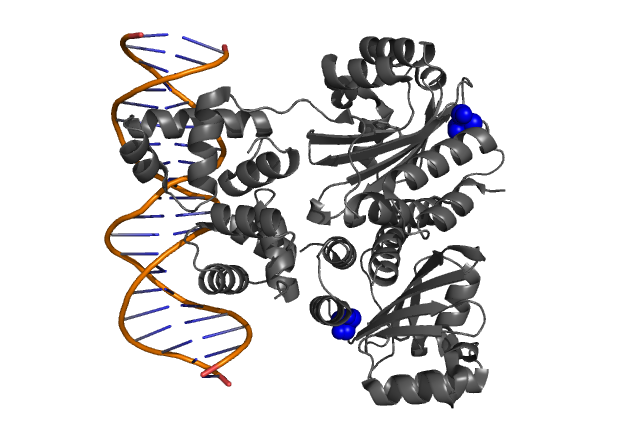
| - | + | It was argued that the presence of the bulky side chain of L42 in LuxR (corresponding to A38 in TraR) | |
| - | + | would cause a reduction in the space available for the acyl side chain thereby significantly reducing the binding affinity to AHL molecules. <br clear="all"> | |
| - | + | ||
| + | {| width="80%" | | ||
| + | |- | ||
| + | | [[Image:LuxR I46F chiba.png|thumb|'''Fig. 1 I45F mutation''']] | ||
| + | | [[Image:LuxR L42S chiba.png|thumb|'''Fig. 2 L42A,S mutation''']] | ||
| + | | style="padding:10px" | '''Fig. 1, Fig. 2''' Hypothetical positions of residues in TraR corresponding to those found to modulate acyl-HSL specificity in LuxR. The crystal structure of the LuxR homologue TraR (PDB 1L3L) has been determined[[Team:Chiba/Experiments:LuxR mutant#Reference|<sup>(3)</sup>]]. | ||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<br clear=all> | <br clear=all> | ||
===Experiment=== | ===Experiment=== | ||
| - | + | '''Mutant construction:'''<br> | |
| + | Using sewing PCR, we introduced each of three above mutations into LuxR. Some of the parts are await for sequence verification. | ||
| - | + | '''Test communication: '''<br> | |
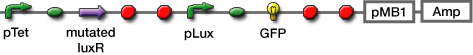
| + | #Transformed sender (Ptet-luxI), mutant LuxR Receiver (Ptet-mLuxR-Plux-GFP) and WT LuxR Receiver into ''E. coli'' strains (JW1908) | ||
| + | #Separately inoculated Sender, WT Receiver (wild type luxR/JW1908) and mutated Receiver (1point mutation/JW1908) in liquid media for 12 h at 37°C. | ||
| + | #Inoculated again in liquid media upto about OD600=2 at 37°C | ||
| + | #Washed, adjusted the cell density, and mixed them. (Sender:Receiver=1000μL:1000μL) | ||
| + | #Incubated at 30°C, measuring the fluorescent intensity intermittently | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{| class="tbl" | | {| class="tbl" | | ||
|- | |- | ||
| Line 65: | Line 61: | ||
[[Image:High-Copy-Receiver Chiba.gif]] | [[Image:High-Copy-Receiver Chiba.gif]] | ||
|} | |} | ||
| + | |||
| + | ===Status (Ongoing)=== | ||
| + | #We have constructed all three mutants described above. | ||
| + | #We haven't seen any noticeable difference in response-time between wt-luxR and I45F (hyper-sensitive) mutant of LuxR. Sucks big time. We think this is probably due to that LuxR is already sensitive enough. | ||
| + | #We have just constructed Leu42 mutants, but they are waiting for the sequence verification (as of Oct 29th). We will conduct the communication experiment as soon as sequencing get completed. Hope we will see the significant time-delay, ideally without any decrease in the endpoint signal intensity. | ||
| + | |||
| + | ===One more plan; Direct Screening for Delay Switcher=== | ||
| + | [[Image:Chiba screening.png|thumb|right|'''Fig. 3 Directed evolution of delay switchers.''']] | ||
| + | We are planning to do a simple directed evolution experiment to isolate the 'delay switcher' mutant of LuxR. | ||
| + | The following is the procedure in our mind. We introduce the random mutation into the entire reading frame of LuxR using error-prone PCR. The mutants plasmid will be transformed with LuxP-gfp plasmid into E coli. Colonies are lifted onto nitrocellulose filter and placed on the top of lawn of Lux-senders. Over time, we keep watching the colony hue. Colonies should turn green when they detect the message passing through the NC filter. We are looking for the colony that turns green significantly after those of wildtype. | ||
| + | <br clear="all"> | ||
===Reference=== | ===Reference=== | ||
| - | #Collins et.al. Directed evolution of Vibrio fischeri LuxR for increased sensitivity to a broad spectrum of acyl-homoserine lactones. Mol. Microbiol. 55, 712–723 (2005) | + | #Collins CH et.al. Directed evolution of Vibrio fischeri LuxR for increased sensitivity to a broad spectrum of acyl-homoserine lactones. Mol. Microbiol. 55, 712–723 (2005) |
| - | #B | + | #Koch B et.al.:The LuxR receptor: the sites of interaction with quorum-sensing signals and inhibitors. Microbiol. 2005 Nov;151(Pt 11):3589-602. |
| + | #Zhang RG ''et. al.'' Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA., Nature, 417, 971-974 (2002) | ||
| + | |||
| + | |||
| + | |||
| + | <!--- | ||
| + | [[Image:Mutant-LuxR Chiba.gif|frame|'''Table 1''' Seisitivity of LuxR mutants. Data modified from Collins et.al. Mol. Microbiol. 55, 712–723 (2005)]] | ||
| + | [[Image:AHL-activation-of-mutagenizenized LuxR molecules Chiba.gif|frame|'''Table 2''' Ahl activation of mutagenized LuxR molecules. '''Data modfied from B.Kock et al. Microbiology (2005), 151, 3589-3602''']]--> | ||
| + | |||
| + | |||
| + | |||
| + | |||
'''>[[Team:Chiba/Project#Receiver|Back to the project page]]''' | '''>[[Team:Chiba/Project#Receiver|Back to the project page]]''' | ||
| + | |||
{| style="color:white;background-color:Maroon" cellpadding="3" cellspacing="3" border="1" bordercolor="white" width="100%" align="center" | {| style="color:white;background-color:Maroon" cellpadding="3" cellspacing="3" border="1" bordercolor="white" width="100%" align="center" | ||
Latest revision as of 09:33, 30 October 2008
| Home | The Team | The Project | Parts Submitted to the Registry | Reference | Notebook | Acknowledgements |
|---|
LuxR mutant (Ongoing)
Design
Probably, the most straightforward approach to make variations in delay time is to create a number of LuxR mutants with different sensitivity. We thought the higher the sensitivity is, the shorter the delay time would be. The lower the sensitivity is, the slower the switch response should be.
At least, two papers on LuxR mutants (1),(2) are available. Among the many candidates, we chose the following three mutations;
- Ile45Phe: This mutation make LuxR 10x more sensitive to Lux-type AHL(1)
- Leu42Ala: Sensitivity (to AHL) becomes 1/15 of the wildtype LuxR(2)
- Leu42Ser: Sensitivity (to AHL) becomes 1/1,000 of the wildtype LuxR(2)
It was argued that the presence of the bulky side chain of L42 in LuxR (corresponding to A38 in TraR)
would cause a reduction in the space available for the acyl side chain thereby significantly reducing the binding affinity to AHL molecules.
| Fig. 1, Fig. 2 Hypothetical positions of residues in TraR corresponding to those found to modulate acyl-HSL specificity in LuxR. The crystal structure of the LuxR homologue TraR (PDB 1L3L) has been determined(3). |
Experiment
Mutant construction:
Using sewing PCR, we introduced each of three above mutations into LuxR. Some of the parts are await for sequence verification.
Test communication:
- Transformed sender (Ptet-luxI), mutant LuxR Receiver (Ptet-mLuxR-Plux-GFP) and WT LuxR Receiver into E. coli strains (JW1908)
- Separately inoculated Sender, WT Receiver (wild type luxR/JW1908) and mutated Receiver (1point mutation/JW1908) in liquid media for 12 h at 37°C.
- Inoculated again in liquid media upto about OD600=2 at 37°C
- Washed, adjusted the cell density, and mixed them. (Sender:Receiver=1000μL:1000μL)
- Incubated at 30°C, measuring the fluorescent intensity intermittently
|
|
| [http://partsregistry.org/Part:BBa_S03623 BBa_S03623] |
|
|
Status (Ongoing)
- We have constructed all three mutants described above.
- We haven't seen any noticeable difference in response-time between wt-luxR and I45F (hyper-sensitive) mutant of LuxR. Sucks big time. We think this is probably due to that LuxR is already sensitive enough.
- We have just constructed Leu42 mutants, but they are waiting for the sequence verification (as of Oct 29th). We will conduct the communication experiment as soon as sequencing get completed. Hope we will see the significant time-delay, ideally without any decrease in the endpoint signal intensity.
One more plan; Direct Screening for Delay Switcher
We are planning to do a simple directed evolution experiment to isolate the 'delay switcher' mutant of LuxR.
The following is the procedure in our mind. We introduce the random mutation into the entire reading frame of LuxR using error-prone PCR. The mutants plasmid will be transformed with LuxP-gfp plasmid into E coli. Colonies are lifted onto nitrocellulose filter and placed on the top of lawn of Lux-senders. Over time, we keep watching the colony hue. Colonies should turn green when they detect the message passing through the NC filter. We are looking for the colony that turns green significantly after those of wildtype.
Reference
- Collins CH et.al. Directed evolution of Vibrio fischeri LuxR for increased sensitivity to a broad spectrum of acyl-homoserine lactones. Mol. Microbiol. 55, 712–723 (2005)
- Koch B et.al.:The LuxR receptor: the sites of interaction with quorum-sensing signals and inhibitors. Microbiol. 2005 Nov;151(Pt 11):3589-602.
- Zhang RG et. al. Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA., Nature, 417, 971-974 (2002)
| Home | The Team | The Project | Parts Submitted to the Registry | Notebook |
|---|
 "
"