Team:Chiba/Experiments:copy number
From 2008.igem.org
(→Result & Discussion) |
|||
| (50 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
<html><link rel="stylesheet" href="https://2008.igem.org/wiki/index.php?title=User:Maiko/chiba.css&action=raw&ctype=text/css" type="text/css" /></html> | <html><link rel="stylesheet" href="https://2008.igem.org/wiki/index.php?title=User:Maiko/chiba.css&action=raw&ctype=text/css" type="text/css" /></html> | ||
| - | [[Image:Chiba-U.gif]] | + | [[Image:Chiba-U.gif|center]] |
{| style="color:white;" cellpadding="3" cellspacing="3" border="0" width="100%" align="center" class="menu" | | {| style="color:white;" cellpadding="3" cellspacing="3" border="0" width="100%" align="center" class="menu" | | ||
!align="center"|[[Team:Chiba|Home]] | !align="center"|[[Team:Chiba|Home]] | ||
| Line 12: | Line 12: | ||
__NOTOC__ | __NOTOC__ | ||
| - | == | + | ==Balancing Player== |
===Design=== | ===Design=== | ||
| - | [[Image:Receiver copy number chiba.jpg|left]] | + | [[Image:Receiver copy number chiba.jpg|left|frame|'''Fig. 1 Player Balancing''']] |
| - | + | ||
| - | + | ||
| - | + | In this section, we intended to create time-delay by altering the | |
| + | amount of luxR protein per cell.(*1) Since the amount of LuxR that | ||
| + | receives AHL differs, we hypothesized that the time required to reach | ||
| + | the threshold AHL concentration will differ. | ||
| + | |||
| + | To do this, we used different copy number plasmids of the Receivers. (*1) | ||
| + | |||
| + | By altering the copy number of plasmids in the receiver cells, the | ||
| + | time required for the response is altered.<BR> | ||
| + | If the copy number is altered, the amount of LuxR produced by the | ||
| + | receivers will also change.<BR> | ||
| + | The amount of LuxR that receives AHL will change, so the time required | ||
| + | for AHL to reach a threshold level will be altered. | ||
| + | |||
| + | |||
<br clear=all> | <br clear=all> | ||
| + | |||
===Experiment=== | ===Experiment=== | ||
| - | + | We used a pMB1 ori high copy vector containing BBa_T9002 and a low | |
| + | copy vector containing BBa_T9002 with a P15A as ori. | ||
| - | + | We determined the effects of altering the plasmid copy number by | |
| + | comparing the above situations. | ||
| - | + | Experiments used the following genetic circuits: | |
| + | <br clear=all> | ||
{| class="tbl" | | {| class="tbl" | | ||
|- | |- | ||
| Line 46: | Line 64: | ||
<br clear=all> | <br clear=all> | ||
| - | + | In addition, a Chloramphenicol resistance marker-containing empty | |
| + | plamid was transformed to the strain containing BBa_T9002, and a | ||
| + | Ampicillin resistance marker-containing empty vector was transformed | ||
| + | into the low copy variant, both resulting in a double transformation. | ||
| + | |||
| + | <br clear=all> | ||
===Method=== | ===Method=== | ||
| - | #Transformed sender(Ptet-LuxI), high copy receiver(Ptet-LuxR-Plux-GFP- | + | #Transformed sender (Ptet-LuxI), high copy receiver (Ptet-LuxR-Plux-GFP-pMB1) and Medium copy receiver (Ptet-LuxR-Plux-GFP-p15A) respectively into ''E coli'' strains (JW1908). |
#Inoculated them independently in liquid media. Incubated at 37°C 12h. | #Inoculated them independently in liquid media. Incubated at 37°C 12h. | ||
#Inoculated again in Fresh liquid media upto about OD600=2 at 37°C | #Inoculated again in Fresh liquid media upto about OD600=2 at 37°C | ||
#Washed sender and receivers. | #Washed sender and receivers. | ||
| - | #Mixed them. (Sender:Receiver= | + | #Mixed them. (Sender:Receiver=1000μL:1000μL) |
#Incubated at 30°C. | #Incubated at 30°C. | ||
| - | #Measured intensity of green fluorescence at regular time intervals.(Fluoroskan AscentR | + | #Measured intensity of green fluorescence at regular time intervals. (Fluoroskan AscentR FL&Fluoroskan AscentR Thermo Electron Corporation) |
| - | + | ||
===Result & Discussion=== | ===Result & Discussion=== | ||
| - | [[Image:Copy-nomber-change-result-detail version Chiba.gif| | + | [[Image:Copy-nomber-change-result-detail version Chiba.gif|frame|left|'''Fig. 2 Time Delay Test: Highcopy Receiver & Reporter vs.Lowcopy Receiver & Reporter''']] |
<br clear=all> | <br clear=all> | ||
| - | |||
| - | + | ||
| - | # | + | Compared to GFP expression from (Ptet-LuxR-plux-GFP-pMB1), expression |
| - | # | + | from (Ptet-LuxR-pLux-GFP-p15A) was greatly reduced (Fig. 2). |
| - | + | ||
| - | + | Possible explanations for this are: | |
| - | + | <BR> | |
| + | #GFP expression was reduced since it was placed on a low copy number plasmid. | ||
| + | #The amount of LuxR synthesized was too small. | ||
| + | However, (Ptet-luxR-p15A + plux-GFP-pMB1) and | ||
| + | (Ptet-LuxR-pLux-GFP-pMB1) resulted in exactly the same trasnfer Curve | ||
| + | (Fig. 3). | ||
| + | |||
| + | Thus, we can deduce that the expression levels of LuxR from the p15A | ||
| + | vector is sufficient to activate pLux from the high copy number | ||
| + | vector. Explanation 1 is therefore more probable. | ||
| + | |||
| + | [[Image:LuxR-high-Low-result Chiba.gif|frame|left|'''Fig. 3 Time Delay Test: Lowcopy Receiver & Highcopy Reporter vs.Highcopy Receiver-Reporter''']] | ||
| Line 75: | Line 107: | ||
<br clear=all> | <br clear=all> | ||
| - | |||
| + | === Demo ~Receivers~ === | ||
| + | Breif explanation on Methods:<br> | ||
| + | 1. LB pre-cultured Sender (BBa_S03623 (JW1908)) was mixed with LB-agar to produce sender containing bacterial plate.<br> | ||
| + | 2. Receiver colony was transfered to a nitrocellulose filter and placed on a Sender (BBa_S03623 (JW1908)) containing bacterial plate.<br> | ||
| + | 3. AHL produced by sender is diffused in the LB-agar plate and the receivers on the nitrocellulose filter synthesize GFP when they are stimulated by a certain amount of AHL.<br> | ||
| + | 4. Plates cultured at 37°C were exposed to UV (312nm) light once every 30 minutes to observe GFP fluorescence. <br> | ||
| + | |||
| + | The receivers are:<br> | ||
| + | *AiiA synthesizing receiver.<br> | ||
| + | *receiver which has different copy number.<br> | ||
| + | *Cross-talk receiver.<br> | ||
| + | |||
| + | |||
| + | ====Results==== | ||
| + | |||
| + | --->more about [[Team:Chiba/Demo_experiments#Demo Experiment ~Receivers~|Receivers Demo experiments detail]] | ||
| + | |||
| + | |||
| + | <br clear=all> | ||
| + | '''>[[Team:Chiba/Project#Receiver|Back to the project page]]''' | ||
| + | <br clear=all> | ||
{| style="color:white;background-color:Maroon" cellpadding="3" cellspacing="3" border="1" bordercolor="white" width="100%" align="center" | {| style="color:white;background-color:Maroon" cellpadding="3" cellspacing="3" border="1" bordercolor="white" width="100%" align="center" | ||
!align="center"|[[Team:Chiba|Home]] | !align="center"|[[Team:Chiba|Home]] | ||
Latest revision as of 09:37, 30 October 2008
| Home | The Team | The Project | Parts Submitted to the Registry | Reference | Notebook | Acknowledgements |
|---|
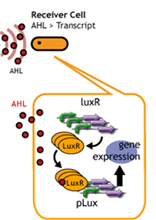
Balancing Player
Design
In this section, we intended to create time-delay by altering the
amount of luxR protein per cell.(*1) Since the amount of LuxR that
receives AHL differs, we hypothesized that the time required to reach
the threshold AHL concentration will differ.
To do this, we used different copy number plasmids of the Receivers. (*1)
By altering the copy number of plasmids in the receiver cells, the
time required for the response is altered.
If the copy number is altered, the amount of LuxR produced by the
receivers will also change.
The amount of LuxR that receives AHL will change, so the time required
for AHL to reach a threshold level will be altered.
Experiment
We used a pMB1 ori high copy vector containing BBa_T9002 and a low copy vector containing BBa_T9002 with a P15A as ori.
We determined the effects of altering the plasmid copy number by comparing the above situations.
Experiments used the following genetic circuits:
|
|
| **[http://partsregistry.org/Part:BBa_S03623 BBa_S03623 (AHL autoinucer)] |
|
|
In addition, a Chloramphenicol resistance marker-containing empty
plamid was transformed to the strain containing BBa_T9002, and a
Ampicillin resistance marker-containing empty vector was transformed
into the low copy variant, both resulting in a double transformation.
Method
- Transformed sender (Ptet-LuxI), high copy receiver (Ptet-LuxR-Plux-GFP-pMB1) and Medium copy receiver (Ptet-LuxR-Plux-GFP-p15A) respectively into E coli strains (JW1908).
- Inoculated them independently in liquid media. Incubated at 37°C 12h.
- Inoculated again in Fresh liquid media upto about OD600=2 at 37°C
- Washed sender and receivers.
- Mixed them. (Sender:Receiver=1000μL:1000μL)
- Incubated at 30°C.
- Measured intensity of green fluorescence at regular time intervals. (Fluoroskan AscentR FL&Fluoroskan AscentR Thermo Electron Corporation)
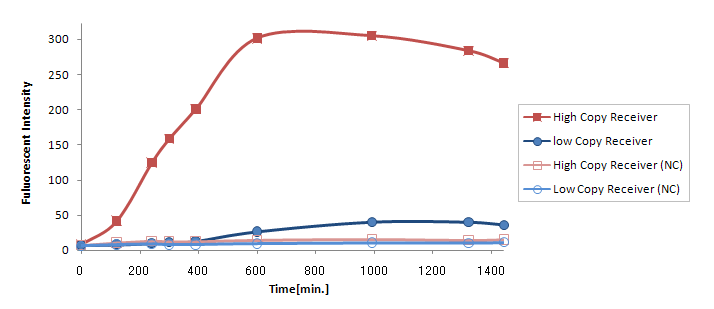
Result & Discussion
Compared to GFP expression from (Ptet-LuxR-plux-GFP-pMB1), expression
from (Ptet-LuxR-pLux-GFP-p15A) was greatly reduced (Fig. 2).
Possible explanations for this are:
- GFP expression was reduced since it was placed on a low copy number plasmid.
- The amount of LuxR synthesized was too small.
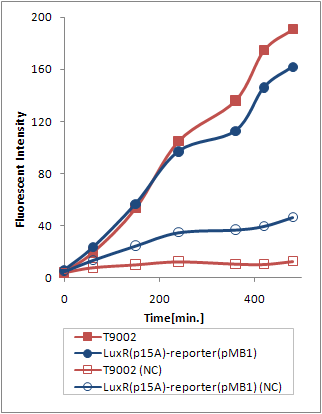
However, (Ptet-luxR-p15A + plux-GFP-pMB1) and (Ptet-LuxR-pLux-GFP-pMB1) resulted in exactly the same trasnfer Curve (Fig. 3).
Thus, we can deduce that the expression levels of LuxR from the p15A vector is sufficient to activate pLux from the high copy number vector. Explanation 1 is therefore more probable.
Demo ~Receivers~
Breif explanation on Methods:
1. LB pre-cultured Sender (BBa_S03623 (JW1908)) was mixed with LB-agar to produce sender containing bacterial plate.
2. Receiver colony was transfered to a nitrocellulose filter and placed on a Sender (BBa_S03623 (JW1908)) containing bacterial plate.
3. AHL produced by sender is diffused in the LB-agar plate and the receivers on the nitrocellulose filter synthesize GFP when they are stimulated by a certain amount of AHL.
4. Plates cultured at 37°C were exposed to UV (312nm) light once every 30 minutes to observe GFP fluorescence.
The receivers are:
- AiiA synthesizing receiver.
- receiver which has different copy number.
- Cross-talk receiver.
Results
--->more about Receivers Demo experiments detail
| Home | The Team | The Project | Parts Submitted to the Registry | Notebook |
|---|
 "
"