Team:Chiba/Project
From 2008.igem.org
| Home | The Team | The Project | Parts Submitted to the Registry | Notebook |
|---|
Contents |
Introduction
- "Team : Chiba - E.coli time manager"
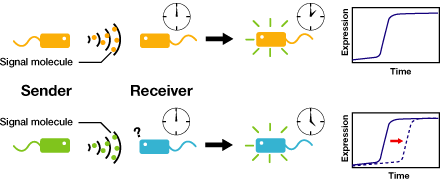
We control the timing of gene expression by using multiple signaling devices.To this end,we utilize molecules associated with Quorum sensing, a phenomenon that allows bacteria to communicate with each other.Our project uses two classes of bacteria: senders and receivers. Senders produce signaling molecules, and Receivers are activated only after a particular concentration of this molecule is reached.Although different quorum sensing species have slightly different signaling molecules, these molecules are not completely specific to their hosts and cross-species reactivity is observed (M.K Winson et al.FEMS Microbiology Letters,1998). Communication using non-endogenous molecules is less sensitive, and requires a higher signal concentration to take effect.This results in slower activation of receivers.
Motivation
Project Design
Project design
Our project uses two classes of bacteria: senders and receivers.Senders produce signaling molecules, and receivers are activated only after a particular concentration of this molecule is reached.The communication using non-endogenous molecules is less sensitive,and it requires higher signal concentration to take effect.This results in slower activation of receivers.
About Quorum Sensing
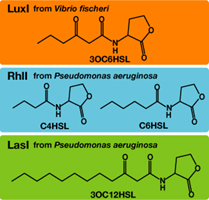
Quorum sensing is a cell-to-cell signaling action of bacteria. They detect the cell density of the same species and coordinate the expression behavior of their cells. Species of Gram-Negative signaling transfer molecules (so-called autoinducer) is a series of acyl homoserine lactone (AHL). The signals are synthesized from S-adenosylmethionine(SAM) by a synthase protein and once they have reached a threshold concentration,they bound to a transcriptional regulatory protein to induce expression of target genes.
More about Quorum Sensing
Controlling the time of a cell-to-cell signaling action
Communication using non-endogenous molecules is less sensitive, and requires a higher signal concentration to take effect.This results in slower activation of receivers.
- Sender Phase
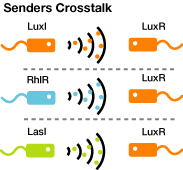
- Quorum-Sensing Cross-talk
AHLs produced by different bacteria differ only in the length of the acyl-chain moiety and substitution at position C-3.(BBa_F2620:Specificity)
- Receiver Phase
- LuxR/Plux mutants show
- a greater response to 3OC6HSL (C. H. Collins.et al.Mol.Microbiol.2005.)
- a increase in sensitivity to 3OC12HSL (B. Koch.et al.Microbiology (2005)).
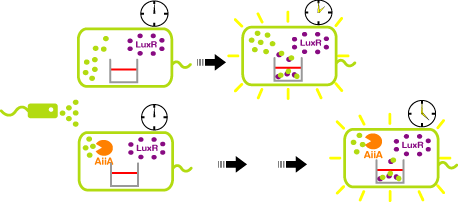
- AHL reporter with aiiA
- Express LuxR and aiiA constantly. AiiA degrades AHL as signaling molecule. Express GFP when the AHL concentration exceed the capacity of aiiA.
- This enables the delay of the activation time of receiver.
About Quorum Sensing
Quorum sensing is a cell-to-cell signaling action of bacteria. They detect the cell density of the same species and coordinate the expression behavior of their cells. Species of Gram-Negative signaling transfer molecules (so-called autoinducer) is a series of acyl homoserine lactone (AHL). The signals are synthesized from S-adenosylmethionine(SAM) by a synthase protein and once they have reached a threshold concentration,they bound to a transcriptional regulatory protein to induce expression of target genes.
More about Quorum Sensing
What our system requires
- Communication module
- Quick output
How Our System Works
Experiments
Quorum-Sensing Cross-talk
- Senders
LuxR/Plux mutants
AiiA Receiver
Method
To characterize quorum sensing crosstalk, constitutive AHL senders were mixed with constitutive receivers and mesure fluorescence intensity.
- Transform Senders into E.coli strains(JW1908/XL10GOLD) and Receiver into E.coli strain(JW1908).
- Inoculated them independently in liquid media. Incubated at 37℃ 12h.
- Washed Senders and receiver.
- Mix them.
- Incubated at 37℃ or 30℃.
- Measured intensity of green fluorescence at regular time intervals.
Result
| Home | The Team | The Project | Parts Submitted to the Registry | Notebook |
|---|
 "
"