Team:Chiba/Receiver experiments
From 2008.igem.org
(Difference between revisions)
(→Method) |
(→Method) |
||
| Line 35: | Line 35: | ||
High Copy ReceiverとMedium Copy Receiverのディレイタイムはfollowing procedureによってanalyzeした。 | High Copy ReceiverとMedium Copy Receiverのディレイタイムはfollowing procedureによってanalyzeした。 | ||
| - | + | #Transformed sender(Ptet-LuxI), high copy receiver(Ptet-LuxR-Plux-GFP-colE1) and Medium copy receiver(Ptet-LuxR-Plux-GFP-p15A) respectively into E coli strains(BD⊿FliC). | |
| - | + | #Inoculated them independently in liquid media. Incubated at 37℃ 12h. | |
| - | + | #Inoculated again in Fresh liquid media upto about OD600=2 at 37℃ | |
| - | + | #Washed sender and receivers. | |
| - | + | #Mixed them. (Sender:Receiver=1000μL:1000μL) | |
| - | + | #Incubated at 30°C. | |
| - | + | #Measured intensity of green fluorescence at regular time intervals.(Fluoroskan AscentR FL&Fluoroskan AscentR Thermo ELECTRON CORPORATION) | |
===Result=== | ===Result=== | ||
Revision as of 06:45, 26 October 2008
| Home | The Team | The Project | Parts Submitted to the Registry | Reference | Notebook | Acknowledgements |
|---|
Contents |
Receiver crosstalk
Method
Result
Change Receiver's Copy Number
レシーバーのコピーナンバーを減らすことで、thresholdに達するまでの時間を短縮する。 (小林)
Method
- High Copy ReceiverBBa_T9002 (AHL Reporter)
- Medium Copy Receiver
High Copy ReceiverとMedium Copy Receiverのディレイタイムはfollowing procedureによってanalyzeした。
- Transformed sender(Ptet-LuxI), high copy receiver(Ptet-LuxR-Plux-GFP-colE1) and Medium copy receiver(Ptet-LuxR-Plux-GFP-p15A) respectively into E coli strains(BD⊿FliC).
- Inoculated them independently in liquid media. Incubated at 37℃ 12h.
- Inoculated again in Fresh liquid media upto about OD600=2 at 37℃
- Washed sender and receivers.
- Mixed them. (Sender:Receiver=1000μL:1000μL)
- Incubated at 30°C.
- Measured intensity of green fluorescence at regular time intervals.(Fluoroskan AscentR FL&Fluoroskan AscentR Thermo ELECTRON CORPORATION)
Result
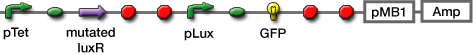
LuxR mutants
Collins et.al. described the hyper-sensitive variants of luxR to AHL.(Collins, C. H., Arnold, F. H. & Leadbetter, J. R. Directed evolution of Vibrio fischeri LuxR for increased sensitivity to a broad spectrum of acyl-homoserine lactones. Mol. Microbiol. 55, 712–723 (2005))
私たちはmutated Receiverを用いることで、AHL感受性の違う2種類(WTと変異体)のレシーバーを用意し、delay-timeのバリエーションを増やした。 (小林)
Method
- Sender
- Receivers
- mutant LuxR Receiver
- WT LuxR Receiver (BBa_T9002 )
LuxR mutantsとWT LuxRのディレイタイムはfollowing procedureによってanalyzeした。
- Transformed sender (Ptet-luxI), mutant LuxR Receiver (Ptet-mLuxR-Plux-GFP) and WT LuxR Receiver ()into E coli strains (BW⊿FliC)
- Inoculated Sender, WT Receiver (wild type luxR/BW⊿FliC) and mutated Receiver (1point mutation/BW⊿FliC) in liquid media for 12 h at 37℃.
- Inoculated again in liquid media upto about OD600=2 at 37℃
- Washed Senders and receiver.
- Mixed them. (Sender:Receiver=1000μL:1000μL)
- Incubated at 30°C.
- Measured intensity of green fluorescence at regular time intervals.
Result
| Home | The Team | The Project | Parts Submitted to the Registry | Notebook |
|---|
 "
"