Team:Chiba/jk/γ/Trl
From 2008.igem.org
(Difference between revisions)
(→Protocol) |
(→Protocol) |
||
| Line 20: | Line 20: | ||
*PBS | *PBS | ||
| - | === | + | ===Method=== |
| - | + | [[Image:Reporter method Chiba.jpg|rifht|thumb|'''Fig. '''レポーターのタイムレスポンスの実験方法]] | |
| - | : | + | Strain:XL10G Kan<sup>R</sup> |
| - | + | ||
| - | + | *Liquid medium experiment | |
| - | + | #Pre-culture | |
| - | + | ##Picked and cultured the following plate in 2mL of LB: | |
| - | + | ###LB-Amp+0.2 % Glucose, (pGFPuv, pLac-Venus YFP, pLac-mCherry, pUC19) | |
| - | : | + | ###LB-Amp, ([http://partsregistry.org/Part:BBa_T9002 BBa_T9002], [http://partsregistry.org/Part:BBa_K084003 BBa_K084003]) |
| - | + | ##Cultured at 37°C for 12h. | |
| - | + | #Culture | |
| - | : | + | ##Dilute pre-cultures and add to new LB medium. |
| - | + | ###LB-Amp+0.2 % Glucose, (pGFPuv, pLac-Venus YFP, pLac-mCherry, pUC19) | |
| + | ###LB-Amp, ([http://partsregistry.org/Part:BBa_T9002 BBa_T9002], [http://partsregistry.org/Part:BBa_K084003 BBa_K084003]) | ||
| + | ##Cultured at 37°C for about 6 h | ||
| + | #Wash | ||
| + | ##Transfer 10mL each of the culture to 50mL centrifuge tubes. | ||
| + | ##Centrifuged for 6min at 3600rpm,20°C and discarded the supernatant. | ||
| + | ##Add physiological saline and resuspention | ||
| + | ##Repeated wash twice. | ||
| + | ##Add M9 minimal medium. | ||
| + | #Mix | ||
| + | ##Dispens each culture into 48-well deep well or 96-well deep well | ||
| + | ##Add IPTG fainal conc. 0.2 mM (pGFPuv, pLac-Venus YFP, pLac-mCherry, pUC19) | ||
| + | ##Add AHL fainal conc. 100nM ([http://partsregistry.org/Part:BBa_T9002 BBa_T9002], [http://partsregistry.org/Part:BBa_K084003 BBa_K084003]) | ||
| + | #Measure fluorescence intensity every 1 h. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<br>'''Result''' | <br>'''Result''' | ||
Revision as of 23:51, 29 October 2008
Contents |
Time Responce Liquid
purpose
インダクションをかけてからいち早く、確認できる出力を見つけること
To find the earliest output gene after induction
装置&試薬(apparatus&reagents)
装置(apparatus)
- しんとう培養器(shaking incubator)(37℃,30℃)
- 48 well plate(deep well)
- Fluoroskan Ascent 2.5(program:Ascent Software Version 2.6)
試薬(reagents)
- AHL(100uM,5uM,100nM)
- IPTG(100nM)
- X-gal
- M9
- PBS
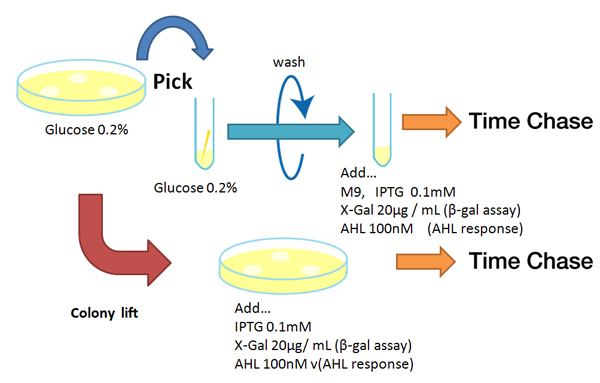
Method
Strain:XL10G KanR
- Liquid medium experiment
- Pre-culture
- Picked and cultured the following plate in 2mL of LB:
- LB-Amp+0.2 % Glucose, (pGFPuv, pLac-Venus YFP, pLac-mCherry, pUC19)
- LB-Amp, ([http://partsregistry.org/Part:BBa_T9002 BBa_T9002], [http://partsregistry.org/Part:BBa_K084003 BBa_K084003])
- Cultured at 37°C for 12h.
- Picked and cultured the following plate in 2mL of LB:
- Culture
- Dilute pre-cultures and add to new LB medium.
- LB-Amp+0.2 % Glucose, (pGFPuv, pLac-Venus YFP, pLac-mCherry, pUC19)
- LB-Amp, ([http://partsregistry.org/Part:BBa_T9002 BBa_T9002], [http://partsregistry.org/Part:BBa_K084003 BBa_K084003])
- Cultured at 37°C for about 6 h
- Dilute pre-cultures and add to new LB medium.
- Wash
- Transfer 10mL each of the culture to 50mL centrifuge tubes.
- Centrifuged for 6min at 3600rpm,20°C and discarded the supernatant.
- Add physiological saline and resuspention
- Repeated wash twice.
- Add M9 minimal medium.
- Mix
- Dispens each culture into 48-well deep well or 96-well deep well
- Add IPTG fainal conc. 0.2 mM (pGFPuv, pLac-Venus YFP, pLac-mCherry, pUC19)
- Add AHL fainal conc. 100nM ([http://partsregistry.org/Part:BBa_T9002 BBa_T9002], [http://partsregistry.org/Part:BBa_K084003 BBa_K084003])
- Measure fluorescence intensity every 1 h.
Result
| ホーム | メンバー紹介 | プロジェクト紹介 | Parts Submitted to the Registry | モデリング | ノート |
|---|
 "
"