Team:Edinburgh/Modelling/Kinetic
From 2008.igem.org
(New page: <div id="header">{{Template:Team:Edinburgh/Templates/Header}}</div> ==Introduction== This section aims to outline the model formulation of the secretion pathway for cellulose degradat...) |
|||
| Line 1: | Line 1: | ||
<div id="header">{{Template:Team:Edinburgh/Templates/Header}}</div> | <div id="header">{{Template:Team:Edinburgh/Templates/Header}}</div> | ||
| - | |||
==Introduction== | ==Introduction== | ||
| - | This section aims to outline the model | + | This section aims to outline the model of the cellulase secretion pathway for cellulose degradation as constructed by the University of |
| - | for cellulose degradation | + | Edinburgh 2008 iGEM team. The computational model includes two operons, 14 species, and 15 reactions, and |
| - | Edinburgh 2008 iGEM team. | + | focusses on the switching on of genes for cellulose degradation by CRP-cAMP. |
| - | + | ||
| - | The computational model includes two operons, 14 species, and 15 reactions, and | + | |
| - | focusses on the switching on of genes for cellulose degradation by CRP. | + | |
| - | + | ||
==Kinetic Modelling== | ==Kinetic Modelling== | ||
| Line 27: | Line 22: | ||
A standard way of modelling (non-spatial) physical systems which has been | A standard way of modelling (non-spatial) physical systems which has been | ||
successfully applied to simulate metabolic systems [Bakker et al., 1997] is the use of | successfully applied to simulate metabolic systems [Bakker et al., 1997] is the use of | ||
| - | Ordinary Differential Equations (ODEs). The aim | + | Ordinary Differential Equations (ODEs). The aim of using ODEs is to describe a biological phenomenon, so that the time dynamics of the system can be analyzed or the steady state values of the variables involved in the model can be calculated. The effects of transcriptional regulation as well as of the changes in concentration of metabolites via enzymes are thus simulated. |
| - | + | ||
| - | involved in the model. | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==The Biological System== | ==The Biological System== | ||
| - | |||
| - | |||
[[Image:Edinburgh-CellLysisModelling.jpg]] | [[Image:Edinburgh-CellLysisModelling.jpg]] | ||
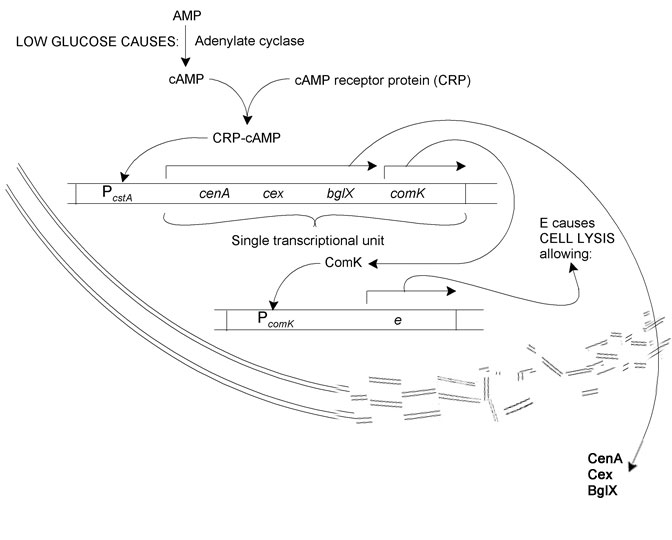
The system we sought to model is as follows: | The system we sought to model is as follows: | ||
| - | Reduction in glucose concentration | + | Reduction in glucose concentration leads to increased adenylate cyclase activity. This results in more AMP being converted to cAMP. |
| - | adenylate cyclase activity. This results in more AMP being converted to cAMP. | + | cAMP binds to CRP. The resultant CRP-cAMP transcription factor activates the transcription of cellulase and cellubiase proteins (cenA, cex, and bglX) and comK via the cstA operator. ComK in turn binds to the comK operator, whereupon phiX174E, a lysis enzyme, is produced. Cell lysis releases the cellulases and bglX into the growth medium. |
| - | cAMP binds to CRP. The resultant CRP-cAMP transcription factor | + | |
| - | + | ||
| - | (cenA, | + | |
| - | operator, whereupon | + | |
| - | releases the cellulases into the growth medium. | + | |
| - | + | ==The Model== | |
| - | + | ||
| - | + | ||
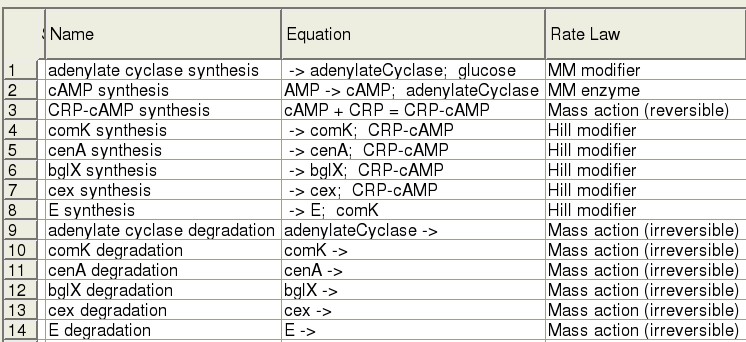
| - | + | Our modelling was done using [http://www.copasi.org/tiki-index.php COPASI]. | |
| - | + | ||
| - | + | [[Image:Edinburgh-ModelTable.jpg]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| + | Our survey of the literature yielded some kinetic information for the genes in our system, in vivo, as well as in E. coli. Parameter values are currently being fit. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| + | ==ODEs== | ||
| - | + | [[Image:Edinburgh-ModelODEs.jpg]] | |
Revision as of 03:25, 30 October 2008
Contents |
Introduction
This section aims to outline the model of the cellulase secretion pathway for cellulose degradation as constructed by the University of Edinburgh 2008 iGEM team. The computational model includes two operons, 14 species, and 15 reactions, and focusses on the switching on of genes for cellulose degradation by CRP-cAMP.
Kinetic Modelling
Through mathematical modelling of cellular processes, we aim to reflect biological function in sets of equations supplied with parameter values that best fit observed reality (Drillon, G. 2008). If the outcomes of model-based simulations closely correspond to experimental results, this indicates that the underlying biological mechanism may be understood. Through the process of modelling, we may generate new hypotheses, as well as investigating doubts on previous suggestions, or corroborating them [Baralla, 2008]. Modelling in preparation and in response to wetlab work can save money and time by focussing on more likely hypotheses.
A standard way of modelling (non-spatial) physical systems which has been successfully applied to simulate metabolic systems [Bakker et al., 1997] is the use of Ordinary Differential Equations (ODEs). The aim of using ODEs is to describe a biological phenomenon, so that the time dynamics of the system can be analyzed or the steady state values of the variables involved in the model can be calculated. The effects of transcriptional regulation as well as of the changes in concentration of metabolites via enzymes are thus simulated.
The Biological System
The system we sought to model is as follows: Reduction in glucose concentration leads to increased adenylate cyclase activity. This results in more AMP being converted to cAMP. cAMP binds to CRP. The resultant CRP-cAMP transcription factor activates the transcription of cellulase and cellubiase proteins (cenA, cex, and bglX) and comK via the cstA operator. ComK in turn binds to the comK operator, whereupon phiX174E, a lysis enzyme, is produced. Cell lysis releases the cellulases and bglX into the growth medium.
The Model
Our modelling was done using COPASI.
Our survey of the literature yielded some kinetic information for the genes in our system, in vivo, as well as in E. coli. Parameter values are currently being fit.
 "
"