Team:Hawaii/Plasmid Prep
From 2008.igem.org
(→Protocol) |
m (→Attempt on 7/23/08) |
||
| (11 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | == Protocol== | |
1. Grow single colony of ''E. coli'' at 37C overnight in 5 ml LB w/ antibiotic selection. <br> | 1. Grow single colony of ''E. coli'' at 37C overnight in 5 ml LB w/ antibiotic selection. <br> | ||
2. Microcentrifuge 1.5 ml cells for 20 sec at 16,000g. Discard supernatant.<br> | 2. Microcentrifuge 1.5 ml cells for 20 sec at 16,000g. Discard supernatant.<br> | ||
| Line 10: | Line 10: | ||
:* 0.2 M NaOH | :* 0.2 M NaOH | ||
:* 1% SDS | :* 1% SDS | ||
| - | 6. Mix by | + | 6. Mix by inverting the tube a few times.<br> |
7. Incubate on ice for 5 min.<br> | 7. Incubate on ice for 5 min.<br> | ||
| + | :* Incubate no more than 5 min. to allow for maximum release of plasmid DNA while minimizing genomic DNA release and overexposure to denaturing conditions. | ||
8. Add 150 μl potassium acetate solution.<br> | 8. Add 150 μl potassium acetate solution.<br> | ||
| + | :* Precipitation of cellular debris may be enhanced by using chilled KOAc. | ||
9. Invert a few times to mix.<br> | 9. Invert a few times to mix.<br> | ||
10. Incubate on ice for 5 min.<br> | 10. Incubate on ice for 5 min.<br> | ||
| Line 24: | Line 26: | ||
''Reference: Short Protocols in Molecular Biology'' | ''Reference: Short Protocols in Molecular Biology'' | ||
| + | |||
| + | * Add 1 μl RNase after step 12. Incubate at 55C for 30-90 min. (Re: SC) | ||
== Results == | == Results == | ||
=== First attempt on 2008.06.17 === | === First attempt on 2008.06.17 === | ||
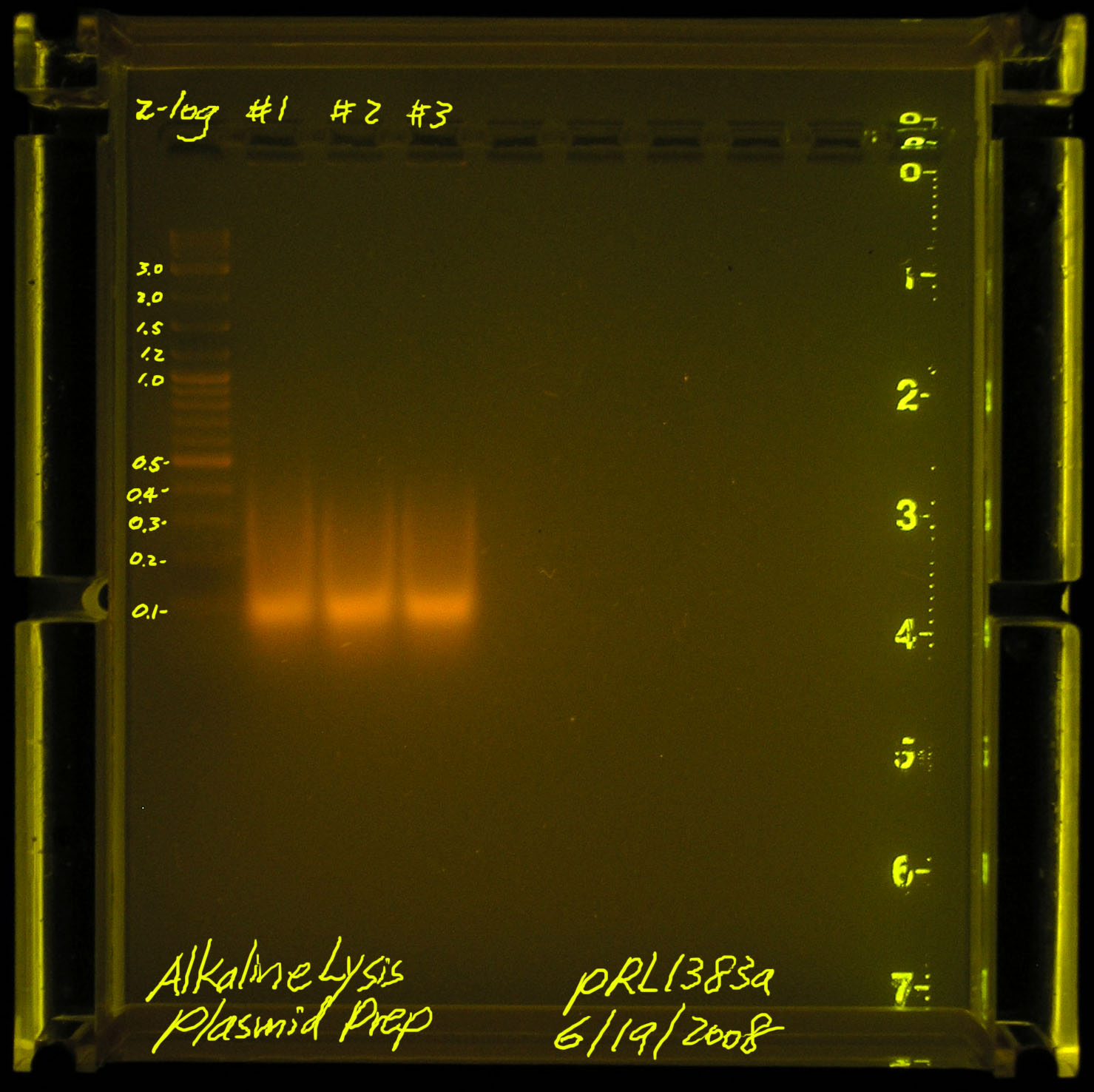
| - | [[Image:20080619-alkaline_lysis_plasmid_prep.jpg|thumb|left|Failed, we should see something bigger than a smear between 100kb-500kb]] | + | [[Image:20080619-alkaline_lysis_plasmid_prep.jpg|thumb|left|Failed, we should see something bigger than a smear between 100kb-500kb, this run uses 10ul plasmid-prep, 2ul loading dye]] |
| - | According to Gernot, we may have sheared the plasmid DNA (he has not seen the picture). Another problem may be that pRL1383a is a LOW copy plasmid. we should start with more material instead of the standard 1.5mL spindown per 30ul of TE suspended final plasmid. | + | According to Gernot, we may have sheared the plasmid DNA (he has not seen the picture). Another problem may be that pRL1383a is a LOW copy plasmid. we should start with more material instead of the standard 1.5mL spindown per 30ul of TE suspended final plasmid.<br/> |
=== Second attempt on 2008.06.19 === | === Second attempt on 2008.06.19 === | ||
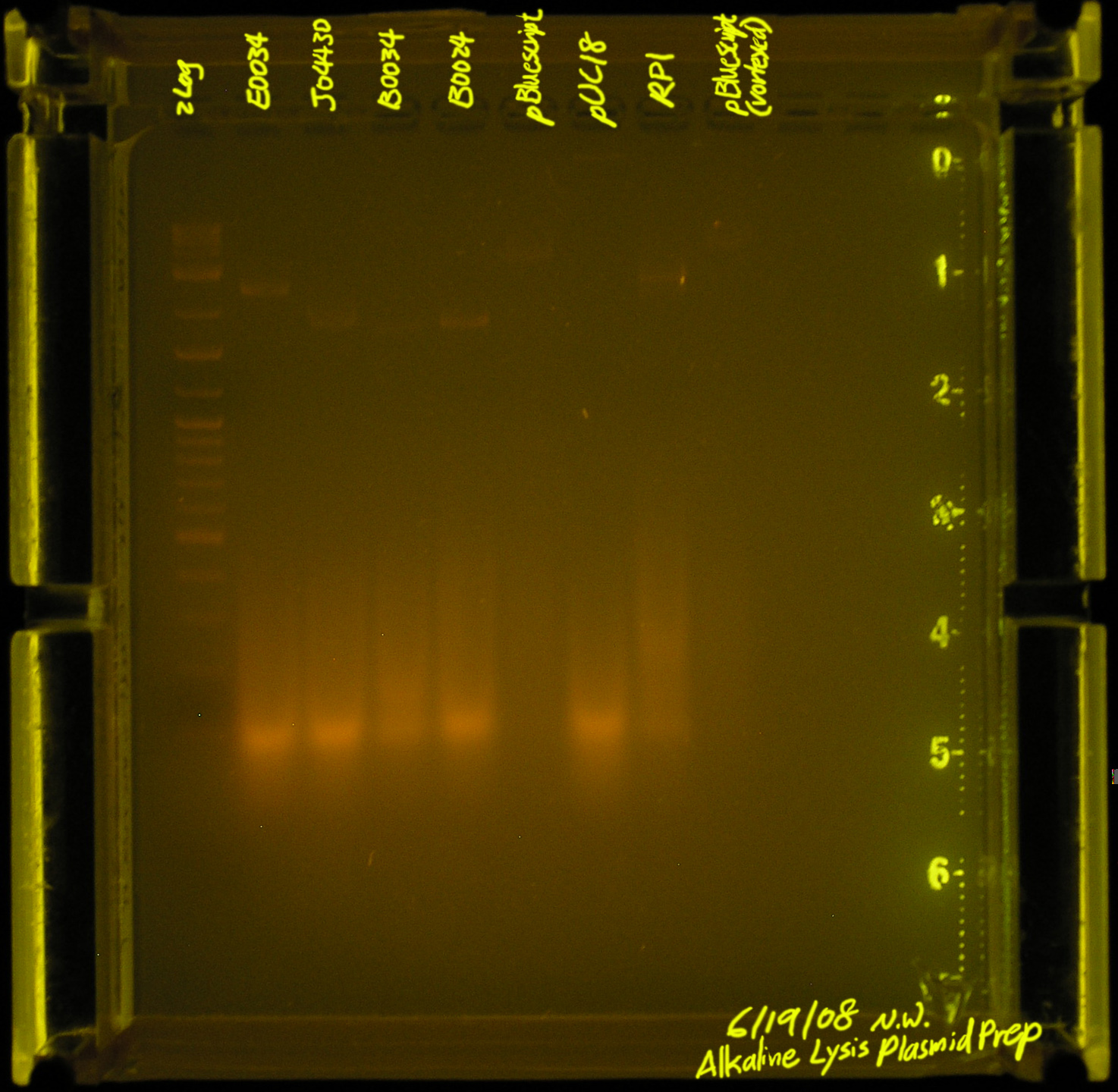
| - | + | [[Image:20080619-alkaline_lysis_plasmid_prep2.jpg|thumb|left|What is that stuff between 100bp and 500bp? this run is half as bright because i'm using 5ul plasmid-prep, 2ul loading dye]] | |
| - | + | ||
| - | + | ||
| - | + | ||
We prepped biobrick plasmids (high copy?) (assuming these clones have the parts we want on them): | We prepped biobrick plasmids (high copy?) (assuming these clones have the parts we want on them): | ||
* E0040 | * E0040 | ||
| Line 40: | Line 41: | ||
* B0034 | * B0034 | ||
* B0024 | * B0024 | ||
| + | Regular Plasmids | ||
| + | * pBluescript | ||
| + | * pUC18 | ||
| + | * RP1 | ||
| + | * pBluescript (vortexed, pippetted up down few times when loading, to see if it's the cause of the small fragments.) | ||
| + | |||
| + | == Discussion == | ||
| + | Plasmid was probably sheared the first time. Band at 100-500bp is likely RNA, rRNA to be specific. To confirm that the band is RNA, we should digest the band with RNase and run it through the gel again. Alternatively, we can try another plasmid prep with RNase added. The Qiagen plasmid prep kit adds RNase in the NaOH/SDS solution. | ||
| + | |||
| + | '' GP suggests we add RNase as early as possible. It will be added alongside GTE.'' | ||
| + | |||
| + | == Attempt on 7/23/08 == | ||
| + | |||
| + | :* Plasmid prep of pSB1A2, pSB1A3, and pSB3K3: 3 1.5 mL preps of the first two, and 10 of the third because it has a lower copy number. Used the protocol from Dr. Callahan's lab ([[http://packrat.stjohn.hawaii.edu/prestinglab/wiki/Protocols]]). | ||
| + | |||
| + | <strong> Results </strong> | ||
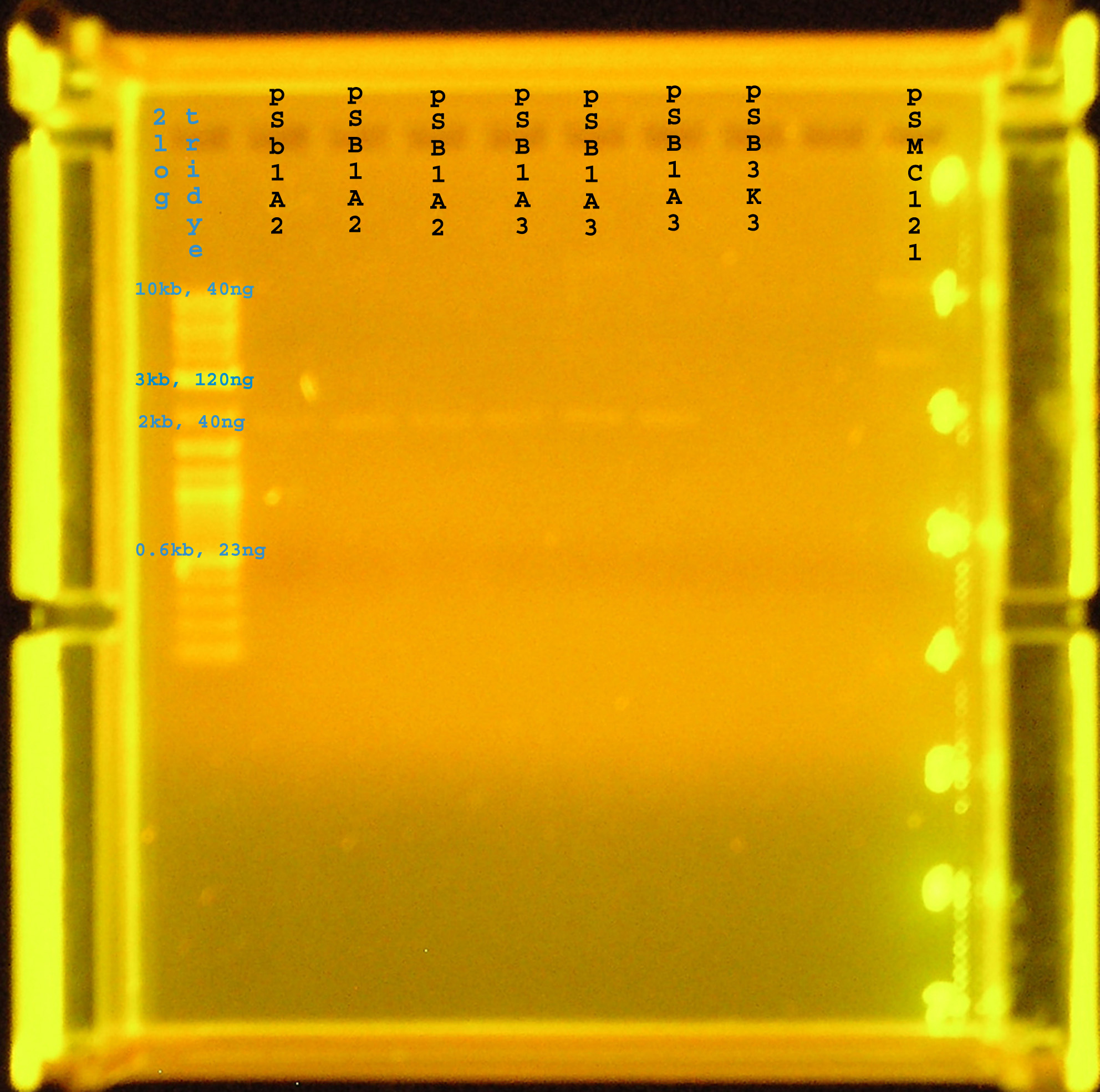
| - | + | [[Image:plasmid_prep.jpg|right|thumb|300px|Plasmid Prep of pSB1A3, pSB1A2, pSB3K3, and a verification of pSMC121.]] | |
| - | == | + | {| border="1" |
| + | |+ '''Plasmid Prep''' | ||
| + | !width="200"|plasmid | ||
| + | !width="200"|size | ||
| + | !width="200"|concentration(gel/nanodrop) | ||
| + | |- | ||
| + | |pSB1A2 | ||
| + | |2kb | ||
| + | |9.2/5.7ng/ul | ||
| + | |- | ||
| + | |pSB1A3 | ||
| + | |2kb | ||
| + | |9.2/5.5ng/uL | ||
| + | |- | ||
| + | |pSB3K3 | ||
| + | |2kb | ||
| + | |0/18ng/uL | ||
| + | |} | ||
| - | + | :*The concentration was also checked using the nanodrop. The concentration is a little different, so for downstream experiments, I am using the conservative number when estimating the amount of DNA to use except in the case of pSB3K3. | |
| - | + | ||
Latest revision as of 01:58, 7 August 2008
Contents |
Protocol
1. Grow single colony of E. coli at 37C overnight in 5 ml LB w/ antibiotic selection.
2. Microcentrifuge 1.5 ml cells for 20 sec at 16,000g. Discard supernatant.
3. Resuspend pellet in 100 μl GTE solution.
- 50 mM glucose
- 10 mM EDTA
- 25 mM Tris-HCl (pH 8.0)
4. Let sit for 5 min. at room temperature.
5. Add 200 μl NaOH/SDS solution.
- 0.2 M NaOH
- 1% SDS
6. Mix by inverting the tube a few times.
7. Incubate on ice for 5 min.
- Incubate no more than 5 min. to allow for maximum release of plasmid DNA while minimizing genomic DNA release and overexposure to denaturing conditions.
8. Add 150 μl potassium acetate solution.
- Precipitation of cellular debris may be enhanced by using chilled KOAc.
9. Invert a few times to mix.
10. Incubate on ice for 5 min.
11. Microcentrifuge for 3 min. at 16,000g.
12. Transfer supernatant to a new tube.
13. Add 0.8 ml 95% ethanol.
14. Incubate for 2 min. at room temperature.
15. Microcentrifuge for 1 min. at 16,000g at room temperature. Remove supernatant.
16. Wash pellet w/ 1 ml 70% ethanol. Aspirate to dry (dry in hood).
17. Resuspend pellet in 30 μl TE buffer.
Reference: Short Protocols in Molecular Biology
- Add 1 μl RNase after step 12. Incubate at 55C for 30-90 min. (Re: SC)
Results
First attempt on 2008.06.17
According to Gernot, we may have sheared the plasmid DNA (he has not seen the picture). Another problem may be that pRL1383a is a LOW copy plasmid. we should start with more material instead of the standard 1.5mL spindown per 30ul of TE suspended final plasmid.
Second attempt on 2008.06.19
We prepped biobrick plasmids (high copy?) (assuming these clones have the parts we want on them):
- E0040
- J04430
- B0034
- B0024
Regular Plasmids
- pBluescript
- pUC18
- RP1
- pBluescript (vortexed, pippetted up down few times when loading, to see if it's the cause of the small fragments.)
Discussion
Plasmid was probably sheared the first time. Band at 100-500bp is likely RNA, rRNA to be specific. To confirm that the band is RNA, we should digest the band with RNase and run it through the gel again. Alternatively, we can try another plasmid prep with RNase added. The Qiagen plasmid prep kit adds RNase in the NaOH/SDS solution.
GP suggests we add RNase as early as possible. It will be added alongside GTE.
Attempt on 7/23/08
- Plasmid prep of pSB1A2, pSB1A3, and pSB3K3: 3 1.5 mL preps of the first two, and 10 of the third because it has a lower copy number. Used the protocol from Dr. Callahan's lab (http://packrat.stjohn.hawaii.edu/prestinglab/wiki/Protocols).
Results
| plasmid | size | concentration(gel/nanodrop) |
|---|---|---|
| pSB1A2 | 2kb | 9.2/5.7ng/ul |
| pSB1A3 | 2kb | 9.2/5.5ng/uL |
| pSB3K3 | 2kb | 0/18ng/uL |
- The concentration was also checked using the nanodrop. The concentration is a little different, so for downstream experiments, I am using the conservative number when estimating the amount of DNA to use except in the case of pSB3K3.
 "
"