Team:Illinois/Antibody RTK Fusion
From 2008.igem.org
| Home | Team | Project | Notebook | Research Articles | Parts | Protocols | Pictures |
Contents |
Core Team Members
Aleem, Joleen, Graham, Palak, Yanfen, Matt
Project Abstract
In the diverse world of cellular signaling pathways one of the most studied and well characterized family of receptors are the receptor tyrosine kinases. These cell surface proteins detect a wide range of extracellular ligands including hormones, growth factors, and cytokines. Receptor tyrosine kinases are most typically composed of an extracellular ligand binding domain, a single transmembrane helix, and an intracellular effector domain. Upon receptor binding of its ligand, signal cascades are activated within the cell. The first step in these signal cascades is often receptor oligomerization and cross-phosphorylation which leads to activation of MAP kinase cascades or other pathways. We plan to leverage the well understood mechanisms of receptor tyrosine kinases for the purpose of detecting the cholera toxin.
Specific Plans, Supplies, and Protocols
We have chosen yeast as our platform for this project due to it's ability to correctly express cell surface proteins. Yeast do not naturally produce receptor tyrosine kinases, but mammalian RTKs have been functionally expressed in yeast. Our first step will be to express in yeast a well understood receptor tyrosine kinase, vascular endothelial growth factor receptor in this case. From here we will create a fusion protein that will allow us to detect a ligand of our choosing. We have chosen the cholera toxin, for which an antibody against the B subunit has been sequenced. A fusion protein containing this antibody in place of VEGFR's normal ligand binding domain will bind the cholera toxin, and due to the pentameric structure of the B subunit multiple receptors will be able to oligomerize and thus activate a signal cascade. At this point our goal will be to integrate this signalling event into an endogenous signaling pathway, which we will then engineer to activate transcription of a reporter such as GFP.
To implement this project we will need to acquire or clone the genes for our receptor tyrosine kinase, the cholera antibody, and other signaling proteins needed to interface with the yeast pathway. Techniques such as PCR, cloning, making fusion proteins, and yeast culture will be needed. Supplies needed will include the cholera B subunit, yeast culture media, and standard molecular biology reagents/kits.
This project can be easily modularized into several subprojects. Expression of the RTK can be carried out independently of ligand binding or signalling, and this could possibly be tested by using fluorescently tagged antibodies to check for cell surface localization of the RTK. The fusion protein could be constructed and antigen binding tested perhaps by FRET or some method of affinity chromatography. The signal cascade could be tested using the unmodified RTK and detecting whether our reporter gene is activated in the presence of the RTK's natural ligand.
In Pictures
Protocols
Literature References
Crystal Structure at 1.7 Å Resolution of VEGF in Complex with Domain 2 of the Flt-1 Receptor
Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis
Structure and sequence of TE33 anti-cholera antibody
Biodistribution and tumor imaging of an anti-CEA single-chain antibody–albumin fusion protein
Single Chain Antibody (SCA) Encoding Genes: One-Step Construction and Expression in Eukaryotic Cells
Planned Labwork, Procedures, and Results
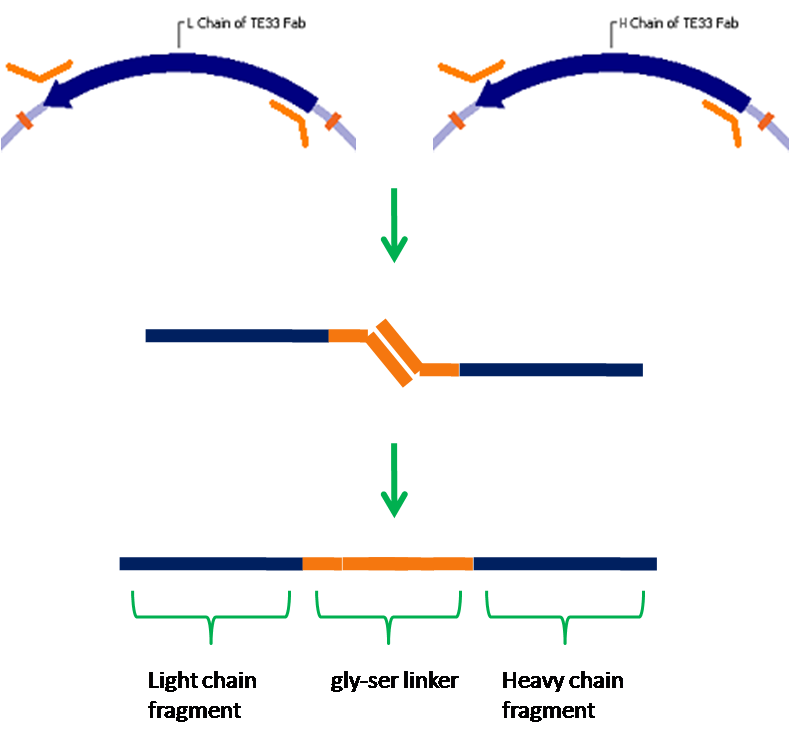
- The relevant fragments of the TE33 antibody have been isolated.
- These fragments have been assembled into a single-chain antibody.
| Home | Team | Project | Notebook | Research Articles | Parts | Protocols | Pictures |
 "
"