Team:Imperial College/Biobricks
From 2008.igem.org
m |
|||
| Line 8: | Line 8: | ||
{{Imperial/Box1|Summary of Data| | {{Imperial/Box1|Summary of Data| | ||
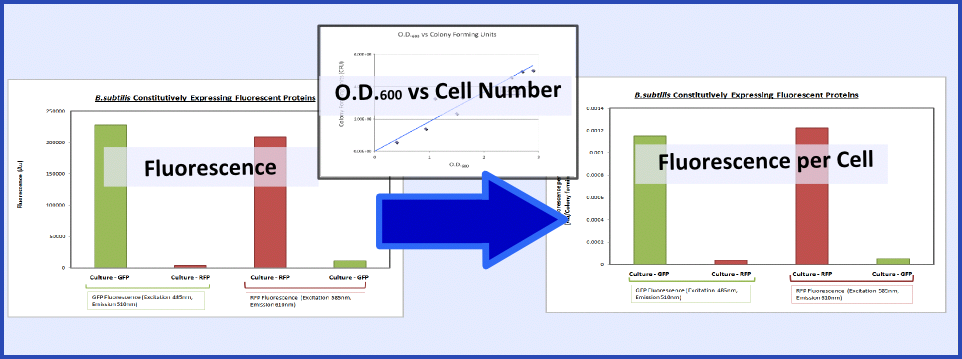
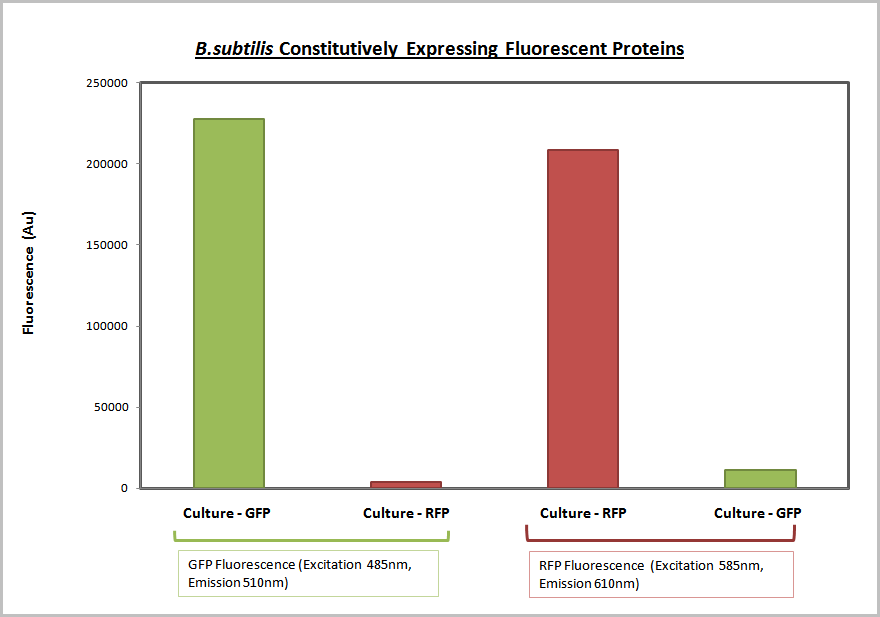
| - | The promoter and RBS combinations were characterised by measuring the | + | The promoter and RBS combinations were characterised by measuring the expression of fluorescent proteins in ''B.subtilis''. Cultures of ''B.subtilis'' transformed with the test constructs and non-transformed ''B.subtilis'' (control) were grown to the mid-log phase. Fluorescence and O.D.<sub>600</sub> were both measured using a plate reader to generate fluorescence levels of the various samples. To make this data more generic the fluorescence data was normalized based on cell number using the O.D.<sub>600</sub> and a '''calibration curve of O.D.<sub>600</sub> vs colony forming units''', as explained in the diagram below: |
| + | <br> | ||
| + | [[Image:Calibration Picture.PNG|600px|center]] | ||
Revision as of 21:40, 29 October 2008
|
||||||||
 "
"