Team:Imperial College/Biobricks
From 2008.igem.org
m |
|||
| Line 2: | Line 2: | ||
<br> | <br> | ||
{{Imperial/Box2|Biobrick Characterisation in ''B.subtilis''| | {{Imperial/Box2|Biobrick Characterisation in ''B.subtilis''| | ||
| - | This year Imperial College has added 45 new ''B.subtilis'' specific parts to the registry. In addition we | + | This year Imperial College has added 45 new ''B.subtilis'' specific parts to the registry. In addition we sought to characterise some of these parts, including the constitutive promoter and ribosome binding site '''pveg-spoVG'''. The testing constructs we developed for this are shown below: |
[[Image:Biobricks Imperial.PNG|center|500px]] | [[Image:Biobricks Imperial.PNG|center|500px]] | ||
}} | }} | ||
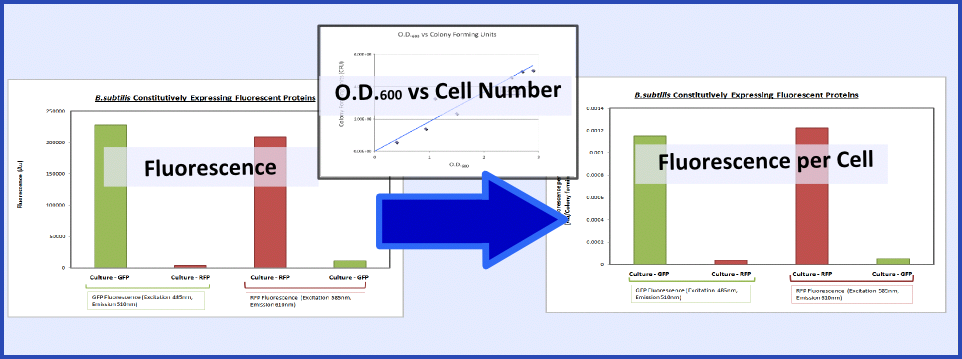
| - | {{Imperial/Box1|Summary of Data| | + | {{Imperial/Box1|Summary of Data|The promoter and RBS combinations were characterised by measuring the expression of fluorescent proteins in ''B.subtilis''. Cultures of ''B.subtilis'' transformed with the test constructs and non-transformed ''B.subtilis'' (control) were grown to the mid-log phase. Fluorescence and O.D.<sub>600</sub> were both measured using a plate reader to generate fluorescence levels of the various samples. To make this data more generic, the fluorescence data were normalized based on cell number using the O.D.<sub>600</sub> and a '''calibration curve of O.D.<sub>600</sub> vs colony forming units''', as explained in the diagram below: |
| - | The promoter and RBS combinations were characterised by measuring the expression of fluorescent proteins in ''B.subtilis''. Cultures of ''B.subtilis'' transformed with the test constructs and non-transformed ''B.subtilis'' (control) were grown to the mid-log phase. Fluorescence and O.D.<sub>600</sub> were both measured using a plate reader to generate fluorescence levels of the various samples. To make this data more generic the fluorescence data | + | |
<br> | <br> | ||
[[Image:Calibration Picture.PNG|600px|center]] | [[Image:Calibration Picture.PNG|600px|center]] | ||
<br> | <br> | ||
| - | This calibration curve allowed us to produce the final data as shown below. This clearly shows ''B.subtilis'' transformed with | + | This calibration curve allowed us to produce the final data as shown below. This clearly shows that ''B.subtilis'' transformed with BioBricks GFP and RFP are fluorescing at the corresponding excitation and emission wavelengths. |
<br> | <br> | ||
[[Image:Fluorescence B.subtilis.PNG|400px|center]] | [[Image:Fluorescence B.subtilis.PNG|400px|center]] | ||
Revision as of 22:38, 29 October 2008
|
||||||||
 "
"