Team:Imperial College/Genetic Circuit
From 2008.igem.org
| Line 24: | Line 24: | ||
<br> | <br> | ||
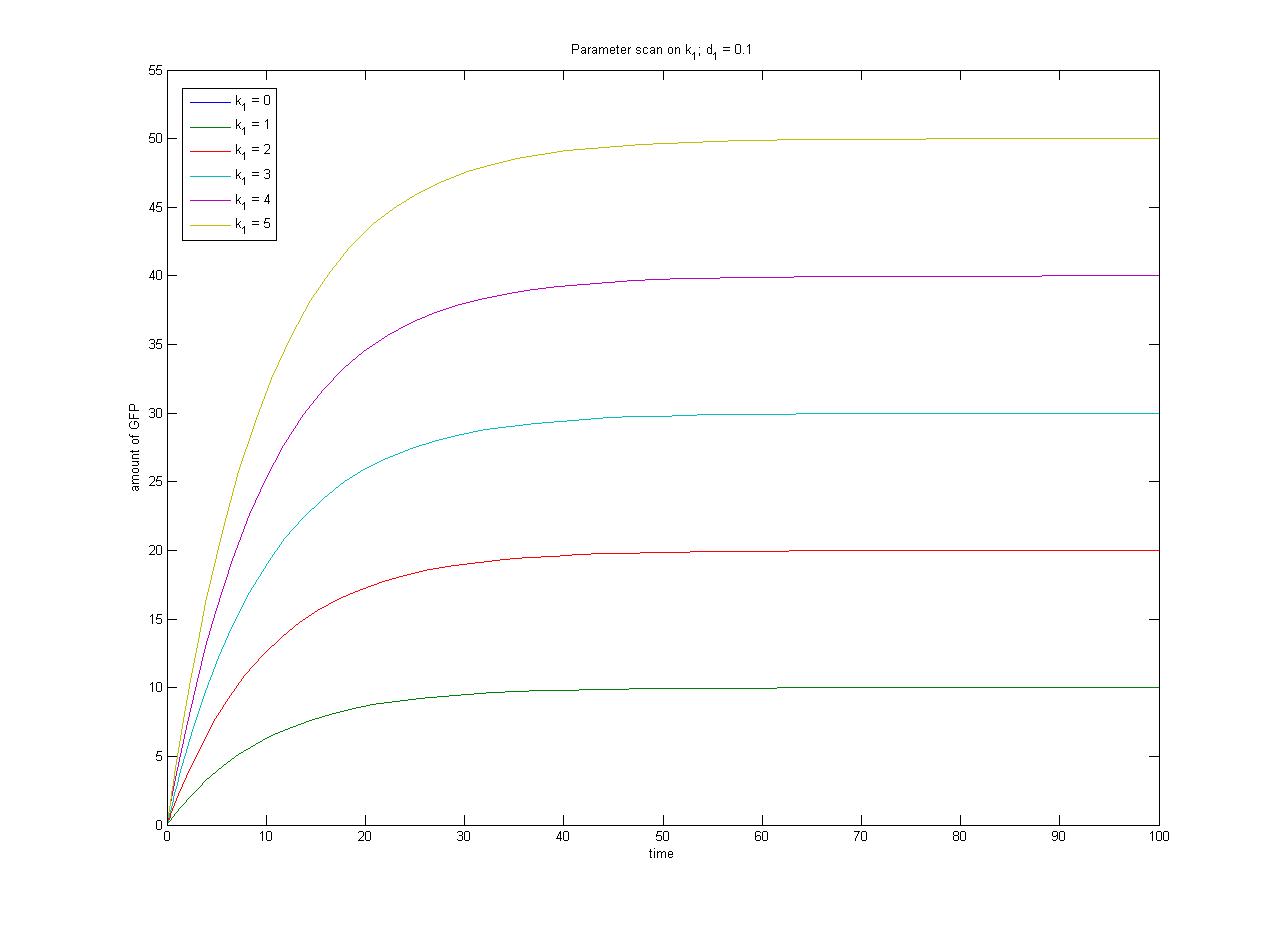
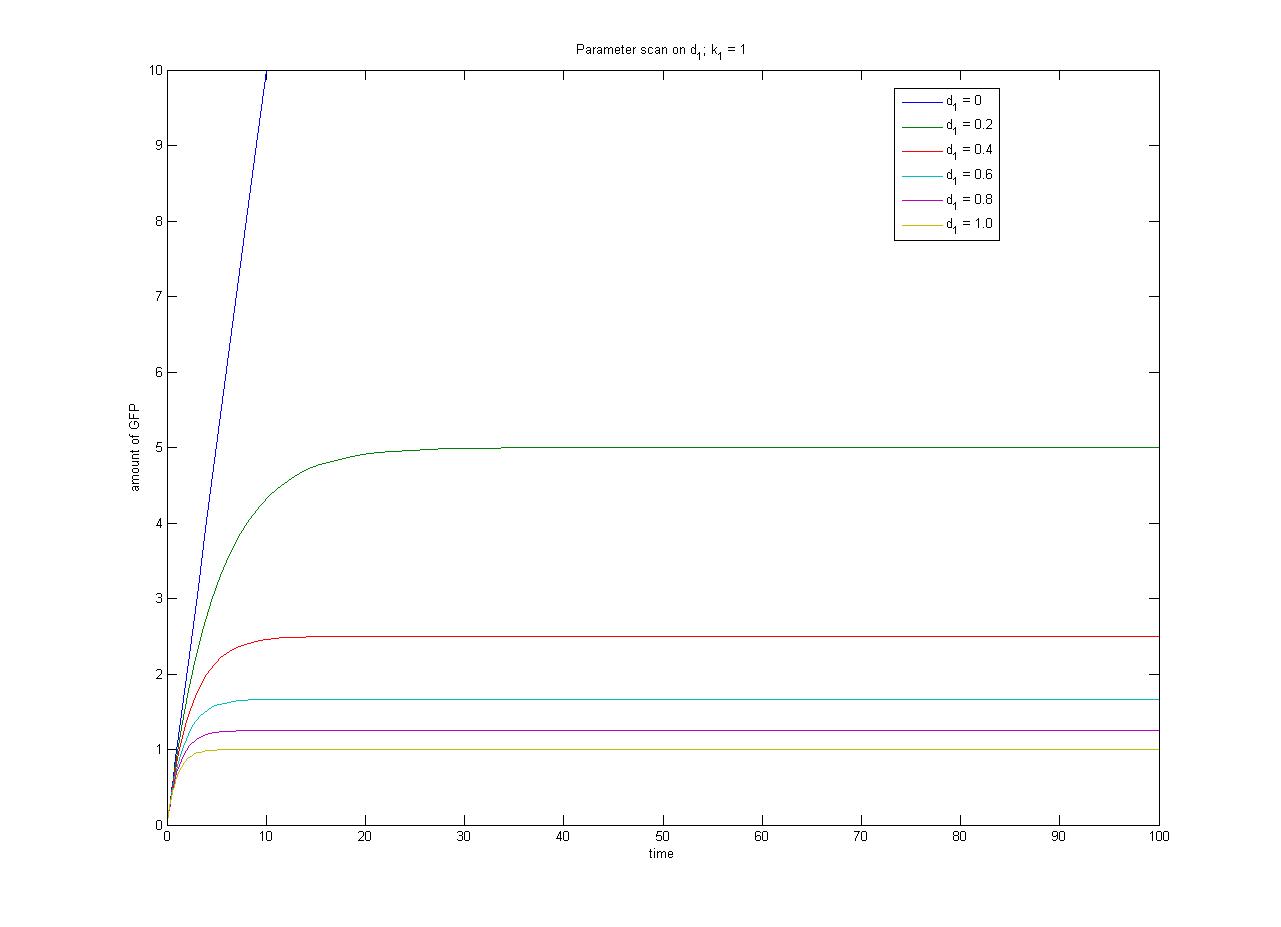
Note from the parameter scan graphs:<br> | Note from the parameter scan graphs:<br> | ||
| - | *In the case where | + | *In the case where k1 {{Equals}} 0, no GFP is sythesised. |
| - | *In the case where | + | *In the case where d1 {{Equals}} 0, the concentration of protein does not reach a steady state. |
|[[Image:Phase 1.PNG|thumb|300px|Constitutive expression of antibiotic resistance (AB) and GFP. GFP brick is part E0040, GFPmut3b. Terminator is part B0015, the double-stop.]] | |[[Image:Phase 1.PNG|thumb|300px|Constitutive expression of antibiotic resistance (AB) and GFP. GFP brick is part E0040, GFPmut3b. Terminator is part B0015, the double-stop.]] | ||
Revision as of 14:56, 21 October 2008
Modelling the Genetic Circuit
|
|||||||||||||||||
 "
"