Team:Imperial College/Genetic Circuit
From 2008.igem.org
| Line 69: | Line 69: | ||
[[Media:InduciblePromoter_SimpleODE.m|ODEs]]<br> | [[Media:InduciblePromoter_SimpleODE.m|ODEs]]<br> | ||
[[Media:InduciblePromoterSimple.m|Simulation File]] | [[Media:InduciblePromoterSimple.m|Simulation File]] | ||
| + | |||
| + | [[Image:SimpleModel.jpg]] | ||
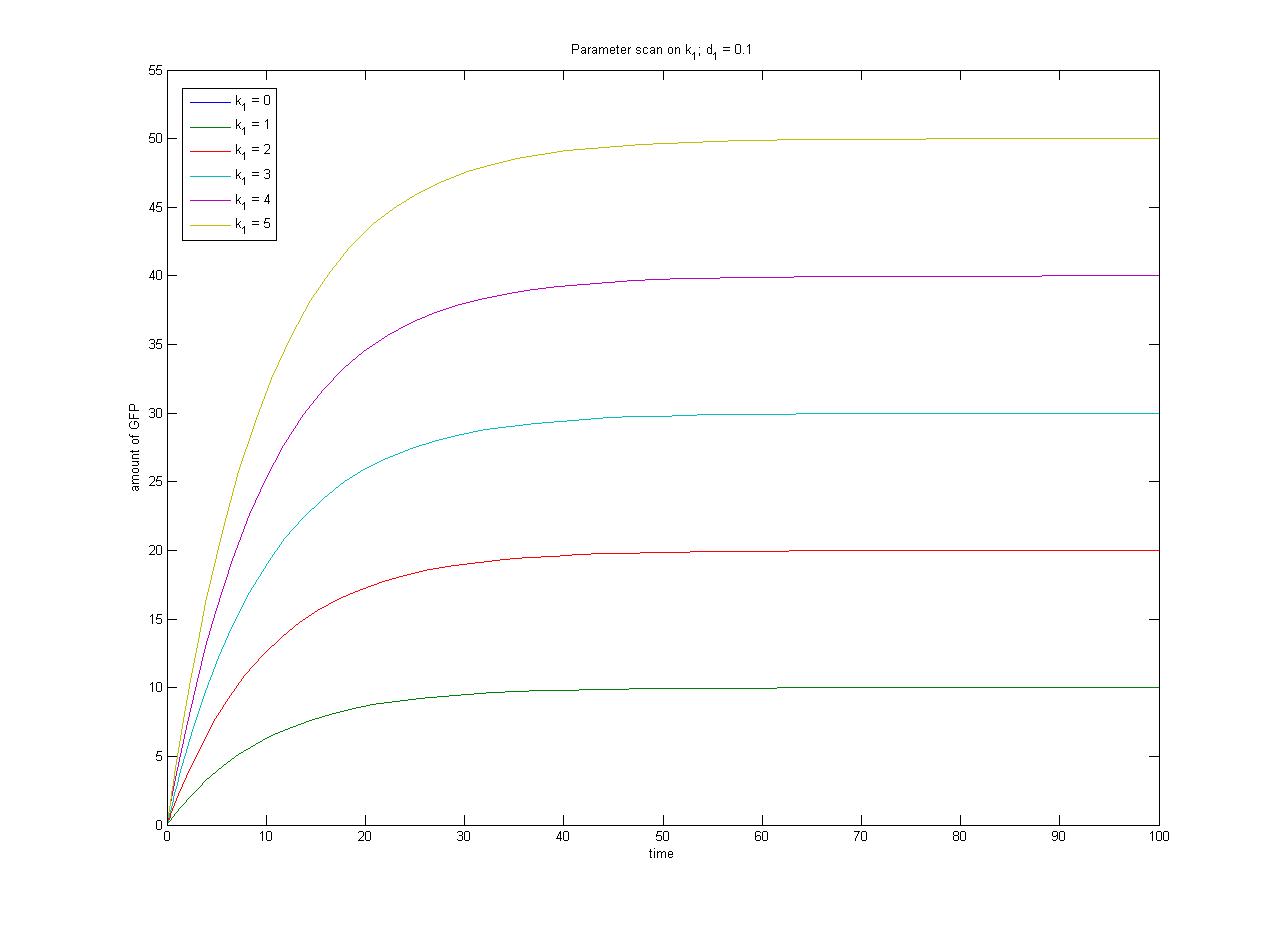
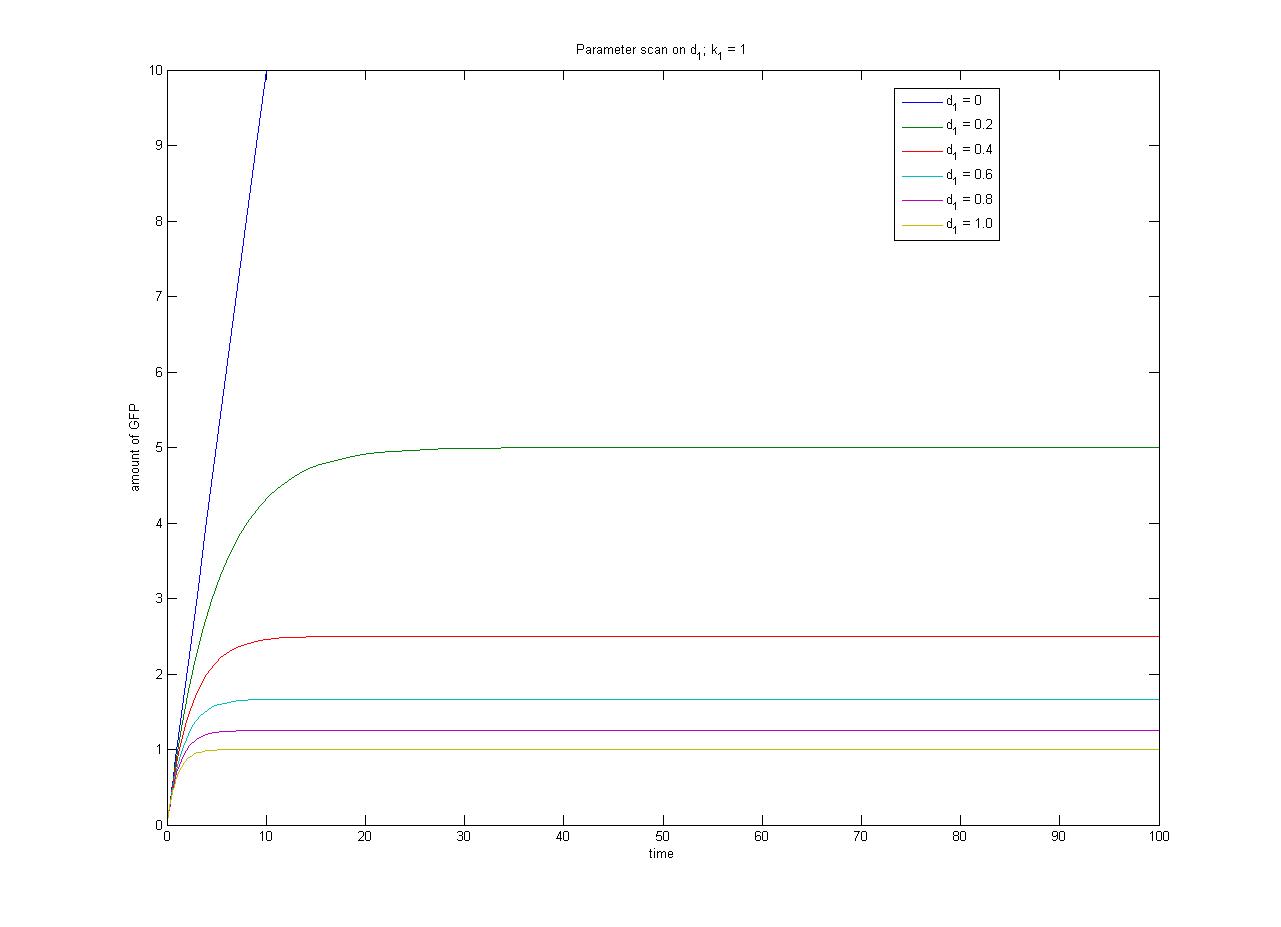
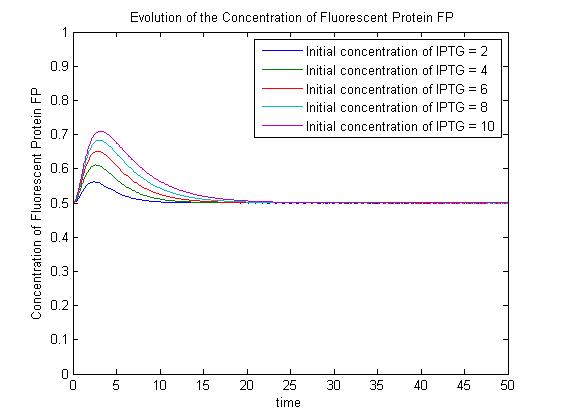
The pre-steady-state dynamic behaviour of the GFP concentration will differ with different initial concentrations of IPTG (but the steady-state behaviour will not). Hence, accuarate data collection during the pre-steady-state phase is crucial for paramater estimation. | The pre-steady-state dynamic behaviour of the GFP concentration will differ with different initial concentrations of IPTG (but the steady-state behaviour will not). Hence, accuarate data collection during the pre-steady-state phase is crucial for paramater estimation. | ||
Revision as of 15:37, 21 October 2008
Modelling the Genetic Circuit
|
|||||||||||||||||||||
 "
"