|
A literature search revealed two models of IPTG-induced expression through the Plac promoter.
In the simpler model (2) IPTG competes with free promoter for LacI binding, but does not itself bind to the LacI-promoter complex.
Assuming this model, all else equal, the steady-state concentration of free promoter and hence the steady-state concentration of GFP are independent of the initial concentration of IPTG.
ODEs
Simulation File
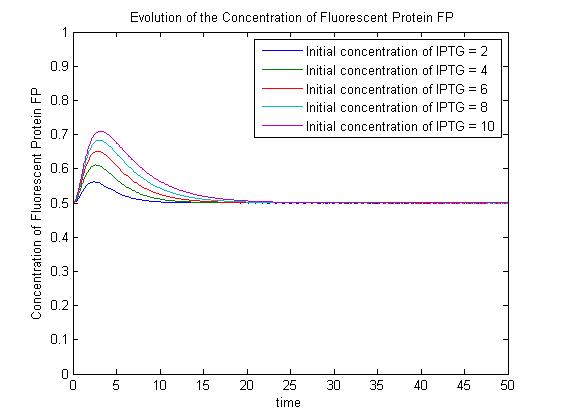 Pre-steady-state time evolution of GFP using the simple model. The pre-steady-state dynamic behaviour of the GFP concentration will differ with different initial concentrations of IPTG (but the steady-state behaviour will not). Hence, accuarate data collection during the pre-steady-state phase is crucial for paramater estimation.
A more sophisticated model allows for interaction between IPTG and the promoter-LacI complex (3). Under this model, the dynamic behaviour (whether or not [GFP] attains a maximum higher than its steady-state value) depends on the relative strengths of the kinetic constants describing the interactions underlying the model. Either way, all else equal the steady-state [GFP] will vary as a Hill-function dependent on the initial concentration of IPTG; this characteristic can be used to discriminate between the two models.
ODEs
Simulation File
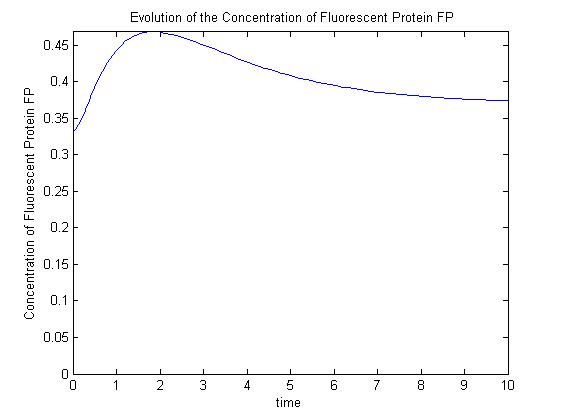 Pre-steady-state time evolution of GFP using the more sophisticated model.
|