From 2008.igem.org
(Difference between revisions)
|
|
| Line 71: |
Line 71: |
| | |}} | | |}} |
| | | | |
| - | {{Imperial/EndPage|Protocols|}} | + | {{Imperial/EndPage|Protocols|Protocols}} |
Latest revision as of 17:44, 28 October 2008
|
| Characterization of Light Inducible Expression
|
Aims
The aim of this protocol is to characterise the light induced expression of GFPmut3b under the control of the pctc and pgsbi promoters. In addition to expression of these constructs, we require overexpression of the YtvA receptor protein. The complexity of the characterisation of light inducible promoters means that we need to carry out several levels of testing:
- Test the overexpressed YtvA proteins are functional,
- Test the exposure time required to induce expression,
Issues to be resolved:
- The stationary phase of growth,
- How to stop the cultures being exposed to light,
- If we Cannot excite the light receptor with the flourometer then what should we use? Could try to filter white light out using filters or cellophane or alternatively we could excite using intense white light.
1. Testing overexpression of YtvA receptor
Equipment
Reagents and Materials
- 2x5ml LB media in 50ml flasks,
- 2x10ml LB media in 100ml flasks,
- pipettes,
- cuvettes,
Protocol
Day 1
- Pick a colony from a plate of B. subtilis and inoculate 5ml of LB media and grow overnight at 37oC.
- Pick a colony from a plate of B. subtilis transformed with construct (define when list is up) and inoculate 5ml of LB media and grow overnight at 37oC.
- Both flasks should be totally covered in foil to ensure that they are grown in darkness.
Day 2
- Measure the O.D.600 of both the overnight cultures using LB media as a blank. With the O.D.600 calculate the dilution to achieve an O.D.600 of 0.5 in 10ml (does not matter too much what O.D.600 we choose, key is to standardise them) using the following calculation:
- Volume of Overnight Culture (X) = (0.5/O.D.600)*10ml
- Volume of fresh Culture (Y) = 10-X (Overnight Culture)
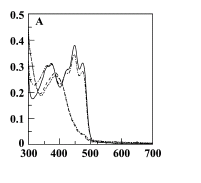
- To determine whether the YtvA light receptor has been expressed correctly we can measure the absorption of the YtvA within each of the cultures. We should see an increase in absorption within the spectra of YtvA overexpressed culture (Figure 1).
- The following wavelengths have been chosen across the absorption spectra of the YtvA:
- 375nm (An Absorption Peak)
- 450nm (Absorption Max)
- 500nm (Not absorbed)
- 550nm (Not absorbed)
- To measure each of these, 1ml of the cultures should be pipetted into a cuvette and measured immediately in a spectrometer, with each wavelength being repeated twice.
- What we should observe is an increase in absorption in the B. subtilis expressing YtvA in the absorption spectra and not in the wavelengths out of the spectra.
2.Testing the exposure time required to induce expression
Equipment
Reagents and Materials
- 2x10ml LB media in 100ml flasks
- Pipettes
- Cuvettes
- 96 well plate
- Plate lids
Protocol
Day 1
- Collect 10ml of LB media (containing suitable antibiotics) into a 100ml flask. Inoculate the media with a single colony from a B. subtilis plate and grow overnight at 37o.
- Grow this culture overnight in the dark by covering the flask in foil.
Day 2
- Collect 1 x 100ml flasks containing 10ml of LB media (containing suitable antibiotics) and remove 1ml of the media and pipette into a blank cuvette (this is to make the blank for OD measurements). Remove 1ml of the overnight culture and measure the OD600
- Mix thoroughly and remove 1ml of the culture and measure the OD600. Now we need to dilute the culture down to a suitable OD600 of 0.2 in 10ml of culture, use the following calculation:
- Volume of Overnight Culture (X) = (0.5/O.D.600)*10ml
- Volume of fresh Culture (Y) = 10-X (Overnight Culture)
- Using the calculated volumes inoculate 1 x 10ml LB media in a 100ml flask that is totally covered in foil. Grow in the shaking incubator at 37oC.
- After....hours of growth check the OD600 of the culture using LB as a blank, if the correct OD600 for exponential phase is reached then remove culture from incubator, if it has not then carry on growing for suitable length of time.
- Place into a shaking incubator and grow until it reaches the exponential phase of growth, the time for this should be determined previously when we do the growth curve but always checked by removing 1ml of each culture and measuring the OD600 using LB media as a blank.
- Once the correct OD600 has been reached then pipette 18x200μl of the B. subtilis into a 96 well plate following the plate schematic. In addition pipette 200μl of LB media into the plate. It is key to minimise the light exposure when loading this plate.
- Once the plate has been loaded then carefully place sticky tape onto the top of the plate.
- Place into the plate reader and open the protocol for characterisation of light inducible promoters and run protocol.
- This protocol needs to be set up to use a suitable emission filter to induce the YtvA receptor. In addition we need to explore how the length of this induction and the intensity of this will affect the activation of the YtvA receptor and so we will need to find out a suitable range of these to test.
- After light induction we need to set up the protocol to measure the OD600 and fluorescence (for mRFP1) every 10 minutes for 6 hours.
- Once data is collected dispose of the 96 well plate into the autoclaved rubbish bins (white).
|
|
|
 "
"