Team:Lethbridge CCS/Notebook
From 2008.igem.org
Contents |
July & August
(all team members)
- as time permitted, we spent time with the U of L team to watch, ask questions about, and try techniques we would be using once we got started on our own project
13 Aug 2008
(Peter, Elizabeth, Paul, Marc, Glenda, Nathan)
- planned more specifics for project (which insert to use, etc)
19 Aug 2008
(Peter, Paul, Elizabeth, Marc, Glenda, Nathan)
- Designed primers
2 Sept 2008
(Peter, Paul, Elizabeth, Glenda, Nathan)
- Resuspended our primers (psB1A7 forward and reverse; TetR-GFP forward and reverse) to 100 μM.
- Made 10-fold dilutions of each primer
- Set up and ran PCR on psB1A7
- varied concentration of vector (1 x, 1/10 x, 1/100 x) and annealing temperatures (58°C, 62°C, 66°C), giving nine variations;
- used materials and amounts as per Phusion polymerase kit
- Cycling temperatures and times for PCR
Initial denaturation 98°C 30 sec ___
Denaturation 98°C 10 sec |
Annealing 58/62/66 °C 30 sec |--- 30 cycles
Extension 72°C 1 min ___|
Final extension 72°C 10 min
Hold 4°C hold
3 Sept 2008
(Peter, Paul, Elizabeth, Marc, Glenda, Nathan)
- PCR of pSB1A7 from yesterday worked well; approximate size was expected to be ~2500 bp, which was verified (see below)
- did PCR with TetR-GFP using same 9 samples and cycling times/temperatures as yesterday except temperature gradient was 57°C-61°C-65°
6 Sept 2008
(Peter, Paul, Marc, Glenda, Nathan)
- looked at PCR results from 3 Sept; some extra bands (see picture)
- decided to do Agarose gel purification of pSB1A7 from 2 Sept and TetR-GFP from 3 Sept
- used pooled samples as follows:
- pSB1A7: 1/100x at 62°C,1x and 1/10x at 66°C
- TetR-GFP: all three 1x samples
- used Fermentas 1kb GeneRuler; ran at 1000 V for 25 minutes
- used MinElute Gel Extraction kit to purify
6 - 13 Sept 2008
(Peter, Paul, Elizabeth, Marc, Glenda)
- spent many hours preparing and rehearsing presentation for AGEM
15 Sept 2008
(Peter, Paul, Elizabeth, Marc, Glenda)
- attended and presented at the AGEM conference in Kananaskis
24 Sept 2008
(Peter, Paul, Elizabeth, Glenda, Nathan)
- prepared ligation mixture to recircularize vector
- Ligation mixture:
10x Buffer 1 μL
Vector 3 μL
Ligase 1 μL
Water 5 μL
left overnight
27 Sept 2008
(Peter, Paul, Elizabeth, Marc, Glenda, Nathan)
- cultures grew nicely, but need to do further tests to see if what grew was what we were looking for
- spent some time discussing which buffers, temperatures, times, etc. to use with T4 endonuclease for testing the LIC method and the Klenow fragment of EcoR1 endonuclease for trying the SLIC method
- decided on using Fermentas buffers for T4 and NEB buffer 2 for Klenow; decided we would use same conditions for both: 20 minutes at 37 °C, followed by 10 minutes at 65 °C.
29 Sept 2008
(Paul, Elizabeth, Marc, Glenda, Nathan)
- Nathan had picked four colonies and grew up in 5 mL culture overnight
- Glycerol stocks: for each culture, combined 500 μL sterilized glycerol with 500 μL cell culture & stored in HJ's -80°C freezer
- Plasmid prep:
- spun down 1.4 mL of culture media to pellet cells (1 minute, 14000 rpm)
- followed directions for QIAprep Spin Miniprep Kit to purify plasmid DNA
- DNA Quantification: analyzed 25-fold dilution of each purified pDNA sample using Ultraspec 3300pro UV/visible spectrophotometer (1s integration)
Sample # A @ 260 nm A @ 280 nm DNA Concentration (μg/mL) 1 0.073 0.045 81 2 0.084 0.054 90 3 0.061 0.039 65 4 0.101 0.066 100
- purified pDNA was stored in freezer for later use
30 Sept 2008
(Paul, Elizabeth, Glenda, Nathan)
- did gel electrophoresis on 4 colonies chosen from smear plate of recircularized plasmid; all same length
- treated our vector and insert with polymerase (each were done with both T4 and Klenow)
- T4 treatment: components and amounts
dCTP 3.33 μL dGTP 3.33 μL
T4 Buffer 2 μL T4 Buffer 2 μL
T4 0.4 μL (2 units) T4 0.4 μL
Vector 2.5 μL Insert 2.5 μL
Water 1.77 μL Water 1.77 μL
- Klenow treatment: components and amounts
NEBuffer2 1 μL NEBuffer2 1 μL
Klenow 2 μL Klenow 2 μL
Vector 1 μL Insert 2.5 μL
Water 6 μL Water 4.5 μL
(Smaller amount of vector used as we did not have any more; probably should have used larger volume as
polymerase volume should be 10% of total volume)
- we then did a PCR purification using the Qiagen MinElute Purification kit, followed by quantification on all 4 of the samples prepared above
- at 50x dilution, T4-GFP gave ratio of 1.6 and concentration of 92 ng/μL, while T4-vector gave ratio of 1 and concentration of 42 ng/μL and both Klenow samples gave basically no results - we then repeated the T4-vector at 25x dilution and obtained a ratio of 1.05 and concentration of 54 ng/μL; we also repeated the Klenow samples at 16x dilution, but results were still inconclusive - we decided we would mix the remaining Klenow samples together and run a transformation -NOTE: water was used as blank, rather than EB buffer as ought to have been used
- we also did a EcoRV digestion and then ran a gel
- components and amounts (for each of 4 colonies grown up previously)
DNA 5 μL
Buffer 1 μL
EcoR-V 1 μL
Water 3 μL
4 Oct 2008
(Marc, Glenda, Nathan)
met to discuss why we had not obtained green colonies from our transformation as expected. There was definitely cutting happening from the EcoRV digest, but the bands showed up at the wrong size. Final conclusion was that probably nothing had worked right from step 1! Most likely what we thought was pSB1A7 actually wasn't, a conclusion we came to based on our own funny results, and on some of the results obtained by the U of L team. So we've probably just been seeing the original template plasmid in all our follow up gels, etc.
Decided to start again, this time using pSB1A2 (though it doesn't have the double terminators we would actually like to see), as the U of L team has it and knows it works. So new primers were designed for this, as well as for xylE, a gene the ULeth team is using, which we hope to use to see whether we can really build a 'new' BioBrick using our method.
6 Oct 2008
(Peter, Elizabeth, Glenda, Nathan)
Reviewed Saturday's discussion; checked that primers were correct and sent off order; discussed our logo and ordering of T-shirts; reviewed steps we would have to go through once our new primers arrive
11 Oct 2008
(Peter, Elizabeth, Marc, Glenda)
- Roxanne had already resuspended and diluted our primers (psB1A2 forward and reverse; LIC_xylE forward and reverse) [100 μM stock; 10μM working].
- Set up and ran PCRs on pSB1A2 and LIC_xylE
- for each, we did 1 x, 1/10 x and 1/100 x of our template and a range of annealing temperatures (54°C, 58°C, 62°C for pSB1A2; 48°C, 52°C, 56°C for LIC_xylE), giving nine variations for each PCR;
- used materials and amounts as per Phusion polymerase kit
- Cycling temperatures and times for PCR
Initial denaturation 98°C 30 sec ___
Denaturation 98°C 10 sec |
Annealing gradient as above 30 sec |--- 30 cycles
Extension 72°C 1 min ___|
Final extension 72°C 10 min
Hold 4°C hold
- numbering:
54°C 58°C 62°C
1x 1 4 7
1/10x 2 5 8
1/100x 3 6 9
- ran a 1% agarose gel on pSB1A2 - expected size ~2000 bp. No results. After discussion, we think this may be due to putting in only enough polymerase for one reaction, not 10.
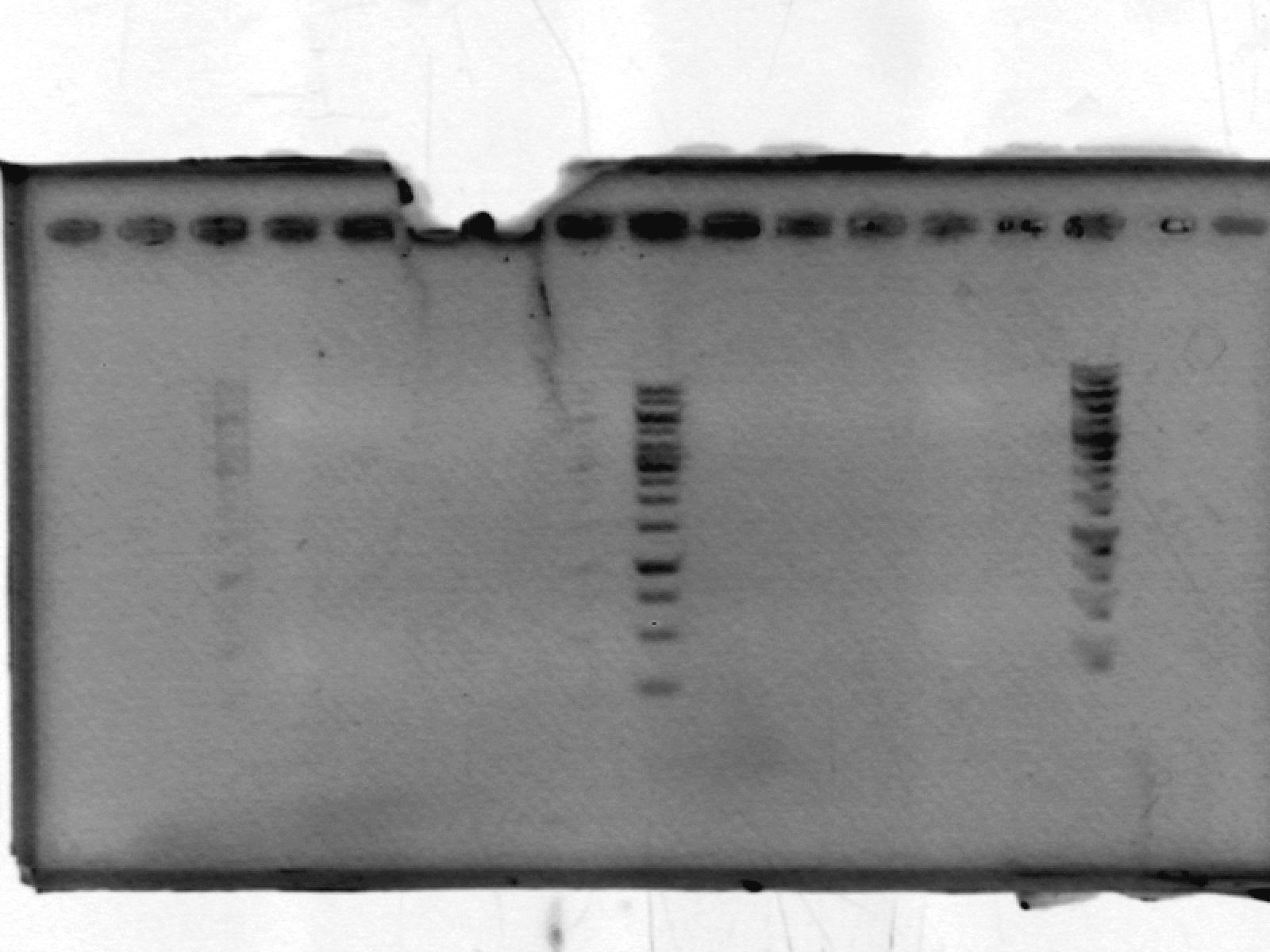 [lanes: 1kb ladder, 1-5, 1kb ladder, 6-9, blank, 1kb ladder]
[lanes: 1kb ladder, 1-5, 1kb ladder, 6-9, blank, 1kb ladder]
- ran a 1% agarose gel on xylE (LIC) - expected size ~1000bp.
 [lanes: 1kb ladder, 1-5, 1kb ladder, 6-9, blank, 1kb ladder]
[lanes: 1kb ladder, 1-5, 1kb ladder, 6-9, blank, 1kb ladder]
13 Oct 2008
(Peter, Marc - yes, we were in the lab on Thanksgiving!)
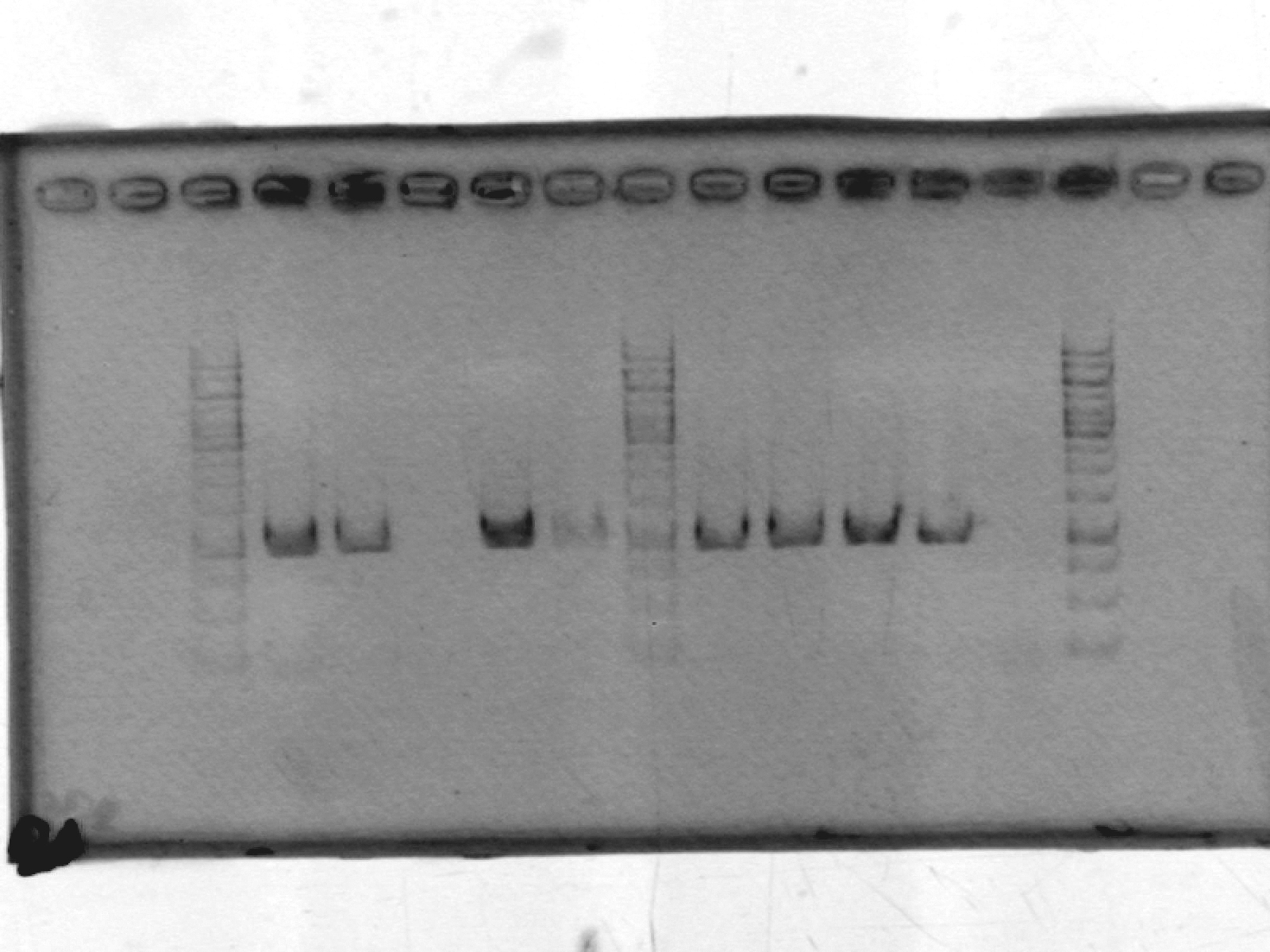
- Re-did PCR of pSB1A2, this time using GFP complete (1) plasmid-prepped by Roxanne.
 [Lanes: 1kb ladder, 1-9, blank, 1kb ladder]
[Lanes: 1kb ladder, 1-9, blank, 1kb ladder]
- Seems to have worked. Possible problem: circular pSB1A2 with GFP complete (~3000bp) may run at the same place as linear pSB1A2 alone (~2000bp). Will run another gel tomorrow to check.
14 Oct 2008
(Marc)
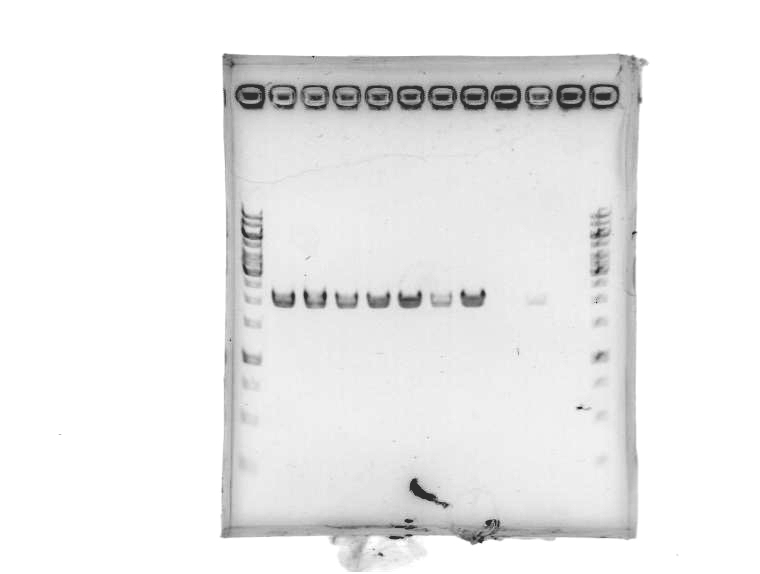
- Ran 1% agarose gel to check where pSB1A2 with GFP complete (template for our pSB1A2 PCR) runs on a gel.
 [Lanes: 1kb ladder, GFP complete (1), #2, #5, #8, 1kb ladder]
[Lanes: 1kb ladder, GFP complete (1), #2, #5, #8, 1kb ladder]
- Unfortunately, template (circular) runs at the same place as our PCR product. We could possibly digest the BioBrick plasmid first; then at least there wouldn't be any competition for transformation.
(Elizabeth, Peter, Glenda)
- Used Fermentas Rapid Ligation kit on our around the world PCR products(1/10, 1/100 concentration, 54°)
-1 μL PCR product 2 μL 5x buffer incubated for 1 hour at room temperature 1 μL ligase 6 μL Water
- Did a transformation of ligated DNA into highly competent cells (using 1 μL ligation mix (1/10, 1/100, pUC19 control)
- left on ice for 30 min
- heat-shock for 30 sec at 42 degrees
- ice for 2 minutes
- add 900 μL SOC medium, 1 hour in shaker at 37 degrees
- plated concentrated and dilute of each of above dilutions
- incubated overnight
- At the U of L iGEM meeting, we discussed our concern about template running at same place as PCR product. HJ recommended doing a DpnI digest since this would cleave the template but leave our PCR product intact. We could then run a gel to compare sizes.
15 Oct 2008
(Glenda, Elizabeth, Peter)
- Interestingly enough, where we had expected to see all or almost all green colonies (if template was present as expected), but in fact there were only white colonies
- Grew up colonies (2 each from our 1/10 and 1/100 dilute plates)
- 5 μL ampicillin, add with colonies to culture
- Incubate at 37°C for 12 hours
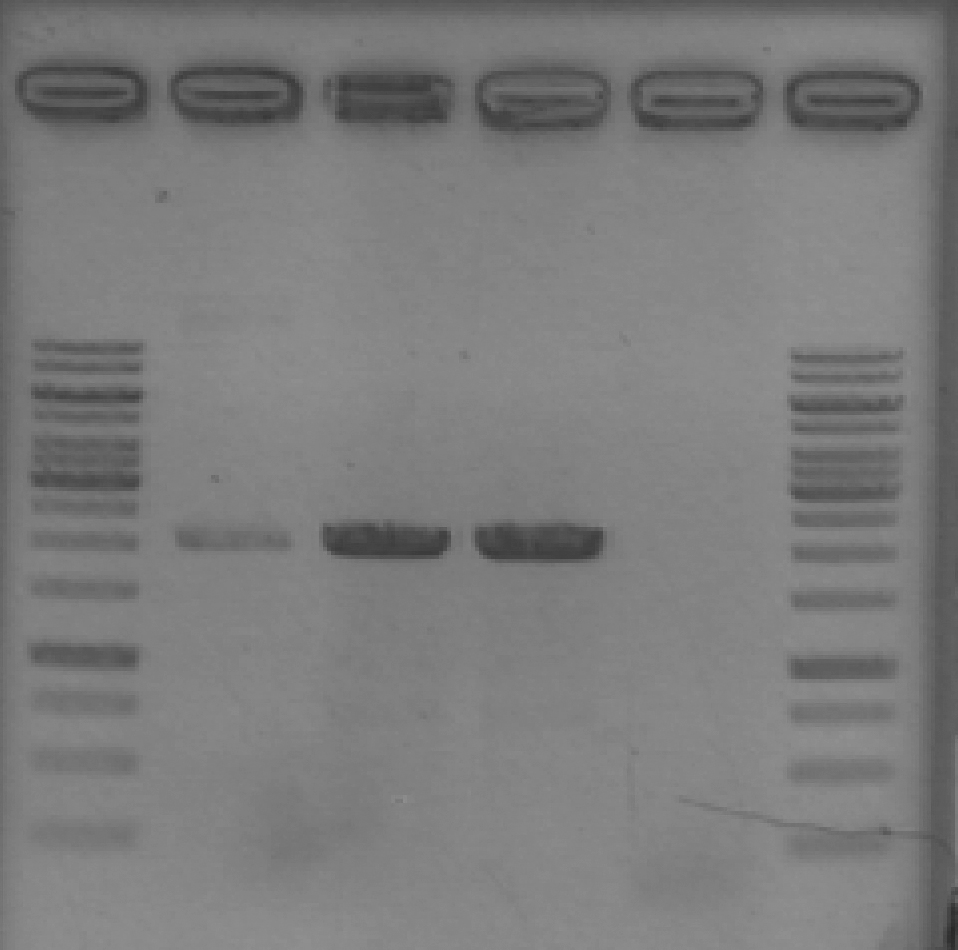
- Set up DpnI digestion
15 μL PCR product/template (used 1x LIC_GFP) 1.75 μL 10x Tango buffer 1 μL DpnI
- Incubated 1 hour at 37°C and then ran a gel
Lane 1: 1 kb GeneRuler ladder Lane 2: GFP with DpnI Lane 3: GFP without DpnI Lane 4: LIC_GFP with DpnI Lane 5: LIC_GFP without DpnI Lane 6: 1 kb GeneRuler ladder
17 Oct 2008
(Glenda, Elizabeth, Peter)
- Set up EcoR-V digest
- components and amounts (for each of 4 colonies grown up previously)
DNA 5 μL
Buffer 1 μL incubated overnight
EcoR-V 1 μL
Water 3 μL
 "
"