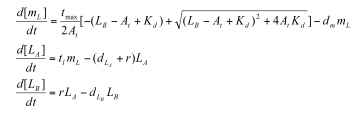Team:Michigan/Project/Modeling/Model1.html
From 2008.igem.org
| Line 18: | Line 18: | ||
*any d= degradation rate of that species | *any d= degradation rate of that species | ||
Professor Daniel Forger came up with this model for the Sequestillator. This simple system assumes the total amount of NifA in the system is constant, and considers three variable: NifL mRNA (mL), NifL (La), and a simple covalent modification of NifL (Lb). The quadratic mRNA production function comes from making rapid equilibrium assumptions (see box to the right of the equations. A= free unbound NifA, L = unbound NifL). From analyzing this small model, we were able to see that in order for oscillations to exist, there needed to be a tight binding between NifL and NifA (i.e., Kd is very small) and one-to-one titration of NifL and NifA (the mRNA production function = tmax*A/At, where A is free NifA in the system. A graph of the function vs. Lb is a oblique line). | Professor Daniel Forger came up with this model for the Sequestillator. This simple system assumes the total amount of NifA in the system is constant, and considers three variable: NifL mRNA (mL), NifL (La), and a simple covalent modification of NifL (Lb). The quadratic mRNA production function comes from making rapid equilibrium assumptions (see box to the right of the equations. A= free unbound NifA, L = unbound NifL). From analyzing this small model, we were able to see that in order for oscillations to exist, there needed to be a tight binding between NifL and NifA (i.e., Kd is very small) and one-to-one titration of NifL and NifA (the mRNA production function = tmax*A/At, where A is free NifA in the system. A graph of the function vs. Lb is a oblique line). | ||
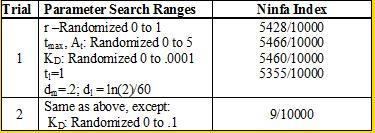
| - | We used the Indexilator to make some "relative" Ninfa index searches. | + | <br> We used the Indexilator to make some "relative" Ninfa index searches. Look at table 1 below: |
<div align='center'>[[Image:chart.png]]<br> | <div align='center'>[[Image:chart.png]]<br> | ||
<b>Table 1</b><br></div> | <b>Table 1</b><br></div> | ||
| - | We see that if we increase our search range for the dissociation constant to 0 to .1, then we get a substantially | + | We see that if we increase our search range for the dissociation constant to 0 to .1, then we get a substantially smaller Ninfa index, illustrating the importance of having a tight binding between NifA and NifL. |
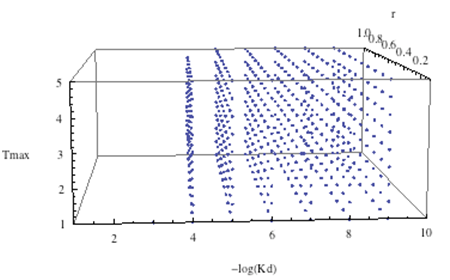
| - | + | <br> Some "sequential" searches (i.e., instead of randomizing, we picked incremental values for each parameters: i.e., one parameter would be varied from 1 to 10 in increments of 1, and another from 1 to 5 in increments of .5. Given below is a three-dimensional cloud picture, of -log(Kd)vs At vs tmax.: | |
| + | <div align='center'>[[Image:cloud.png]]<br> | ||
| + | <b>Table 1</b><br></div> | ||
| + | <br> -log(Kd) was tested at values 1 to 10 in increments of 1 (for some reason, the -log(Kd)=10 values got cut off). | ||
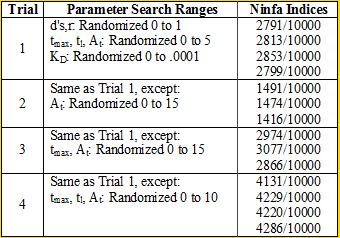
<div align='center'>[[Image:chart2.png]]<br> | <div align='center'>[[Image:chart2.png]]<br> | ||
<b>Table 2</b> <br></div> | <b>Table 2</b> <br></div> | ||
Revision as of 00:54, 30 October 2008
|
|---|
|
Sequestillator Model 1: A simple modelParameters:
Professor Daniel Forger came up with this model for the Sequestillator. This simple system assumes the total amount of NifA in the system is constant, and considers three variable: NifL mRNA (mL), NifL (La), and a simple covalent modification of NifL (Lb). The quadratic mRNA production function comes from making rapid equilibrium assumptions (see box to the right of the equations. A= free unbound NifA, L = unbound NifL). From analyzing this small model, we were able to see that in order for oscillations to exist, there needed to be a tight binding between NifL and NifA (i.e., Kd is very small) and one-to-one titration of NifL and NifA (the mRNA production function = tmax*A/At, where A is free NifA in the system. A graph of the function vs. Lb is a oblique line).
We see that if we increase our search range for the dissociation constant to 0 to .1, then we get a substantially smaller Ninfa index, illustrating the importance of having a tight binding between NifA and NifL.
We again see the necessity of a tight binding between NifA and NifL |
|---|
 "
"