Team:Minnesota/ProjectTimeBomb
From 2008.igem.org
| Home | Team Time Bomb Homepage | Team Page | Project Page | Parts Submitted to the Registry | Team Notebook |
|---|
Overall project
The process of in situ Bioremediation would be aided through the use of microorganisms that undergo synchronized cell death. Bioremediation relies on microorganisms' enzymatic pathways to break down hazard chemicals in the environment. However, in order to protect the environment from the release of a foreign population of microorganisms, most contaminants have to be excavated or pumped off-site (ex situ) before the proper microogranisms can be applied. The transport of contaminants makes ex situ bioremediation just as expensive as the burn or bury methods of waste removal. Although if the remediating microorganisms were engineered to clean up until the contaminant was removed, and then collectively die, their impact on native populations could be reduced significantly. To achieve synchronized apoptosis after a set number of generations we first had be able to link cell divisions to a predictable buildup of toxin. This required that the a promoter regulated by cell cycle state would have to be cloned out of E. coli.
Project Detail
The protein DnaA is required for cell replication and the cell cycle dependent repression of mioC. Replication of the bacterial chromosome is triggered through the highly conserved binding of the DnaA protein to a region called the oriC. However, four of these DnaA binding sites are also found in the promoter of the gene mioC. (2),(3) In this case, the binding of DnaA has been found to repress the promoter of mioC during S-phase. (3)
First Proof of Concept: Linking Cell Division To a Reporter
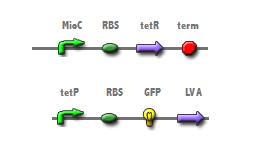
The cell cycle dependent expression of the mioC promoter should accurately count cell division when linked to a reporter. The insertion of mioC into a plasmid downstream from a tetracycline repressor (tetR) will allow for tetR mediated repression of the tetracycline promoter in the bottom construct (Fig 1). Therefore, the expression of GFP from the bottom construct should only occur during each generation's S-phase. To visualize each cell division a short pulse of GFP, conferred by the LVA tag, was thought a preferable means to reduce background noise. To test if cell division is linked to protein expression, synchronization of cell division will be confered by alternating media conditions. (4)
Once it is confirmed that the cell cycle is linked to GFP expression, integration of a toxin-antitoxin feedback loop will be experimented with.
Second Proof of Concept: Incorporation of Antitoxin (MazE) and toxin (MazF) into a Feedback Loop
The toxin-antitoxin gene pair mazEF is involved in bacterial apoptosis. MazF encodes a lethal toxin, whereas MazE encodes a less stable antitoxin to nullify the cellular effect of MazF; therefore, the inhibition of MazE brings about cell death. In wild type E. coli mazEF-mediated cell death is induced through stressful environmental conditions (overcrowding, lack of media, etc.) (5).
If the levels of mazF were slowly increased every cell cycle, cells could undergo a set number of divisions until a threshold level of mazF has been reached, at which point the population would undergo synchronized apoptosis.
 "
"