Team:NYMU-Taipei/Project/Guanidine
From 2008.igem.org
Blackrabbit (Talk | contribs) |
Blackrabbit (Talk | contribs) |
||
| Line 5: | Line 5: | ||
[[Image:NYMU_Gugu2.jpg]] | [[Image:NYMU_Gugu2.jpg]] | ||
| - | <br>'''BACKGROUND:''' <br> Based on the study | + | <br>'''BACKGROUND:''' <br> Based on the study done by Natelson and Sherwin, guanidino compounds can be formed from urea through the metabolization plotted above at pH 7.4. It explains the physiological phenomena why guanidinosuccinate does not appear in the urine in significant amounts except in uremia and why there is no quantitative correlation between guanidinosuccinate concentration in the urine and urea concentration in the serum. |
'''THE POTENTIAL SOLUTION:''' <br> We need to regulate the guanidine and urea removal together. Applied to pNhaA, the 0CT3 homolog transpoter can remove the guanidino compound when pH sensor activates. Meanwhile, the UreaR promoter sense the amount of urea in the environment and responds to it by degrading urea into ammonia. We proposed that this alkaline product could neutralize the acidic environment by bonding with the outward-pumped protons and thereby regulating the pH to strengthen the cleansing of guanidine. | '''THE POTENTIAL SOLUTION:''' <br> We need to regulate the guanidine and urea removal together. Applied to pNhaA, the 0CT3 homolog transpoter can remove the guanidino compound when pH sensor activates. Meanwhile, the UreaR promoter sense the amount of urea in the environment and responds to it by degrading urea into ammonia. We proposed that this alkaline product could neutralize the acidic environment by bonding with the outward-pumped protons and thereby regulating the pH to strengthen the cleansing of guanidine. | ||
{{:Team:NYMU-Taipei/Footer}} | {{:Team:NYMU-Taipei/Footer}} | ||
Revision as of 16:29, 2 August 2008
| Home | Project Overview: | pH Sensor | Attachment | Time Regulation | Waste Removal | Experiments and Parts | About Us |
MOTIVATION:
Renal failure can make one unable to regulate water and electrolyte balances, and metabolic waste. If not treated, lethargy, weakness, shortness of breath, and generalized swelling may occur.
LITERATURE:
Proposed Mechanism for Urea Nitrogen Re-Utilization: Relationship between Urea and proposed Guanidine cycles
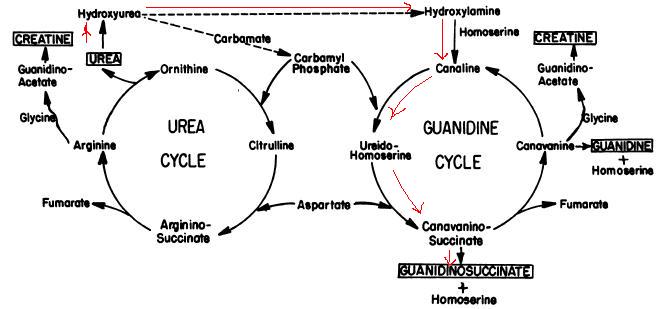
BACKGROUND:
Based on the study done by Natelson and Sherwin, guanidino compounds can be formed from urea through the metabolization plotted above at pH 7.4. It explains the physiological phenomena why guanidinosuccinate does not appear in the urine in significant amounts except in uremia and why there is no quantitative correlation between guanidinosuccinate concentration in the urine and urea concentration in the serum.
THE POTENTIAL SOLUTION:
We need to regulate the guanidine and urea removal together. Applied to pNhaA, the 0CT3 homolog transpoter can remove the guanidino compound when pH sensor activates. Meanwhile, the UreaR promoter sense the amount of urea in the environment and responds to it by degrading urea into ammonia. We proposed that this alkaline product could neutralize the acidic environment by bonding with the outward-pumped protons and thereby regulating the pH to strengthen the cleansing of guanidine.
 "
"
