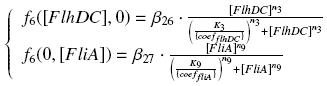|
|
| Line 1: |
Line 1: |
| - | [[Image:f3env.png|thumb]] (see [[Team:Paris/Modeling/Oscillations#Biochemical_Assumptions|the considerations on the use of EnvZ]])
| + | {{Paris/Menu}} |
| | | | |
| - | We have [EnvZ]<sub>produced</sub> = {coef<sub>env</sub>}''expr(pTet)'' = {coef<sub>env</sub>} ƒ1([aTc]<sub>i</sub>)
| + | {{Paris/Header|Method & Algorithm : ƒ6}} |
| | | | |
| - | and [EnvZ]<sub>total</sub> = [EnvZ]<sub>b</sub> + [EnvZ]<sub>produced</sub>
| + | [[Image:f6DCA.png|thumb|Specific Plasmid Characterisation for ƒ6]] |
| | | | |
| - | and [FliA] = {coef<sub>FliA</sub>}''expr(pBad)'' = {coef<sub>FliA</sub>} ƒ2([arab]<sub>i</sub>)
| + | We have <span style="color:#0000FF;">[''FlhDC'']<sub>''real''</sub> = {coef<sub>''flhDC''</sub>} ƒ1([aTc]<sub>i</sub>)</span> |
| | + | and <span style="color:#0000FF;">[''FliA'']<sub>''real''</sub> = {coef<sub>''fliA''</sub>} ƒ2([arab]<sub>i</sub>)</span> |
| | | | |
| - | So, if we denote phosphorylated OmpR by ''OmpR<sup>*</sup>'', we have
| + | but we use <span style="color:#0000FF;">[aTc]<sub>i</sub> = Inv_ƒ1( [''FlhDC''] ) </span> |
| | + | and <span style="color:#0000FF;">[arab]<sub>i</sub> = Inv_ƒ2( [''FliA''] ) </span> |
| | | | |
| - | [[Image:F3ompfromenv.jpg|center]]
| + | So, at steady-states, |
| | | | |
| - | that we can then introduce in the previous expression ([[Team:Paris/Modeling/f3|see ƒ3]]) :
| + | [[Image:F6.jpg|center]] |
| | | | |
| - | [[Image:F3ompfinalenv.jpg|center]]
| + | <br> |
| | | | |
| - | <br><br> | + | <div style="text-align: center"> |
| | + | {{Paris/Toggle|Table|Team:Paris/Modeling/More_f6_Table}} |
| | + | </div> |
| | | | |
| - | {|border="1" style="text-align: center"
| |
| - | |param
| |
| - | |signification
| |
| - | |unit
| |
| - | |value
| |
| - | |comments
| |
| - | |-
| |
| - | |[expr(pFlhDC)]
| |
| - | |expression rate of <br> pFlhDC '''with RBS E0032'''
| |
| - | |nM.min<sup>-1</sup>
| |
| - | |
| |
| - | |need for 20 mesures with well choosen values of [aTc]<sub>i</sub>
| |
| - | |-
| |
| - | |γ<sub>GFP</sub>
| |
| - | |dilution-degradation rate <br> of GFP(mut3b)
| |
| - | |min<sup>-1</sup>
| |
| - | |0.0198
| |
| - | |
| |
| - | |-
| |
| - | |[GFP]
| |
| - | |GFP concentration at steady-state
| |
| - | |nM
| |
| - | |
| |
| - | |need for 20 mesures
| |
| - | |-
| |
| - | |(''fluorescence'')
| |
| - | |value of the observed fluorescence
| |
| - | |au
| |
| - | |
| |
| - | |need for 20 mesures
| |
| - | |-
| |
| - | |''conversion''
| |
| - | |conversion ratio between <br> fluorescence and concentration
| |
| - | |nM.au<sup>-1</sup>
| |
| - | |(1/79.429)
| |
| - | |
| |
| - | |}
| |
| | | | |
| - | <br><br> | + | <div style="text-align: center"> |
| | + | {{Paris/Toggle|Algorithm|Team:Paris/Modeling/More_FP_Algo}} |
| | + | </div> |
| | | | |
| - | {|border="1" style="text-align: center"
| + | Then, if we have time, we want to verify the expected relation |
| - | |param
| + | |
| - | |signification <br> corresponding parameters in the [[Team:Paris/Modeling/Oscillations#Resulting_Equations|equations]]
| + | [[Image:SumpFlgA.jpg|center]] |
| - | |unit
| + | |
| - | |value
| + | <br> |
| - | |comments
| + | |
| - | |-
| + | <center> |
| - | |K<sub>21</sub><sup>eff</sup>
| + | [[Team:Paris/Modeling/Implementation| <Back - to "Implementation" ]]| <br> |
| - | |dissociation constant OmpR_-_EnvZ <br> K<sub>21</sub><sup>eff</sup>
| + | [[Team:Paris/Modeling/Protocol_Of_Characterization| <Back - to "Protocol Of Characterization" ]]| |
| - | |nM
| + | </center> |
| - | |
| + | |
| - | |the literature [[Team:Paris/Modeling/Bibliography|[?] ]] gives K<sub>21</sub> =
| + | |
| - | |-
| + | |
| - | |n<sub>21</sub>
| + | |
| - | |complexation order OmpR_-_EnvZ <br> n<sub>21</sub>
| + | |
| - | |no dimension
| + | |
| - | |
| + | |
| - | |the literature [[Team:Paris/Modeling/Bibliography|[?] ]] gives n<sub>21</sub> =
| + | |
| - | |-
| + | |
| - | |{coef<sub>env</sub>}
| + | |
| - | |coefficient due to the difference of the RBS and degradation rate between EnvZ and GFP <br> ! not precised in the equations !
| + | |
| - | |no dimension
| + | |
| - | |
| + | |
| - | |! not precised in the equations ! watch out when writing the corresponding simulating program
| + | |
| - | |-
| + | |
| - | |[EnvZ]<sub>b</sub>
| + | |
| - | |"basal" presence of EnvZ <br> [EnvZ]<sub>b</sub> | + | |
| - | |nM
| + | |
| - | |
| + | |
| - | |the literature [[Team:Paris/Modeling/Bibliography|[?] ]] gives, under high osmolarity, [EnvZ]<sub>b</sub> =
| + | |
| - | |-
| + | |
| - | |[OmpR]<sub>b</sub>
| + | |
| - | |"basal" presence of OmpR <br> [OmpR]<sub>b</sub>
| + | |
| - | |nM | + | |
| - | |
| + | |
| - | |the literature [[Team:Paris/Modeling/Bibliography|[?] ]] gives, under high osmolarity, [OmpR]<sub>b</sub> =
| + | |
| - | |}
| + | |
|
Method & Algorithm : ƒ6
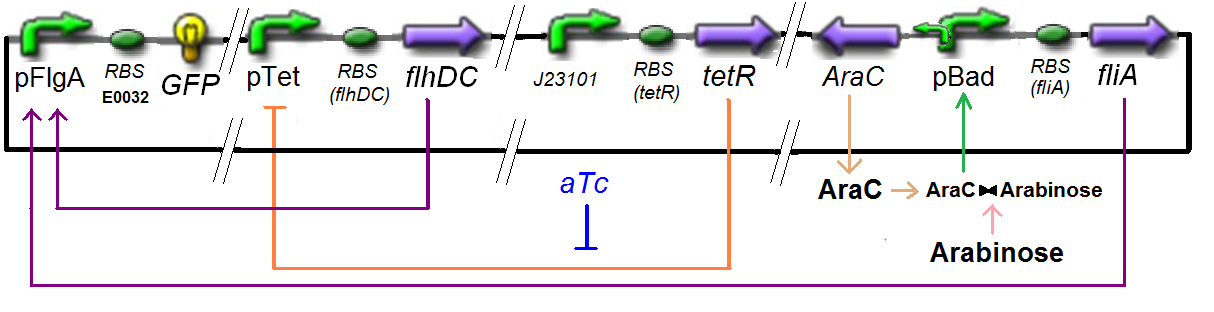 Specific Plasmid Characterisation for ƒ6 We have [FlhDC]real = {coefflhDC} ƒ1([aTc]i)
and [FliA]real = {coeffliA} ƒ2([arab]i)
but we use [aTc]i = Inv_ƒ1( [FlhDC] )
and [arab]i = Inv_ƒ2( [FliA] )
So, at steady-states,
↓ Table ↑
| param
| signification
| unit
| value
| comments
|
| (fluorescence)
| value of the observed fluorescence
| au
|
| need for 20 mesures with well choosen values of [aTc]i
and for 20 mesures with well choosen values of [arab]i
and 5x5 measures for the relation below?
|
| conversion
| conversion ratio between
fluorescence and concentration
↓ gives ↓
| nM.au-1
| (1/79.429)
|
|
| [GFP]
| GFP concentration at steady-state
| nM
|
|
|
| γGFP
| dilution-degradation rate
of GFP(mut3b)
↓ gives ↓
| min-1
| 0.0198
| Time Cell Division : 35 min.
|
| ƒ6
| activity of
pFlgA with RBS E0032
| nM.min-1
|
|
|
| param
| signification
corresponding parameters in the equations
| unit
| value
| comments
|
| β26
| total transcription rate of
FlhDC><pFlgA with RBS E0032
β26
| nM.min-1
|
|
|
| (K3/{coeffliA})
| activation constant of FlhDC><pFliL
K3
| nM
|
|
|
| n3
| complexation order of FlhDC><pFliL
n3
| no dimension
|
|
|
| β27
| total transcription rate of
FliA><pFliL with RBS E0032
β27
| nM.min-1
|
|
|
| (K9/{coefflhDC})
| activation constant of FliA><pFliL
K9
| nM
|
|
|
| n9
| complexation order of FliA><pFliL
n9
| no dimension
|
|
|
|
↓ Algorithm ↑
|
find_ƒP
function optimal_parameters = find_FP(X_data, Y_data, initial_parameters)
function output = act_pProm(parameters, X_data)
for k = 1:length(X_data)
output(k) = parameters(1)*hill(X_data(k), parameters(2), parameters(3));
end
end
options=optimset('LevenbergMarquardt','on','TolX',1e-10,'MaxFunEvals',1e10,'TolFun',1e-10,'MaxIter',1e4);
optimal_parameters = lsqcurvefit( @(parameters, X_data) act_pProm(parameters, X_data),...
initial_parameters, X_data, Y_data, options );
end
|
Then, if we have time, we want to verify the expected relation
<Back - to "Implementation" |
<Back - to "Protocol Of Characterization" |
|
 "
"