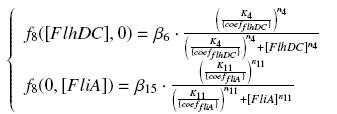Team:Paris/Modeling/f8
From 2008.igem.org
(Difference between revisions)
| Line 1: | Line 1: | ||
{{Paris/Menu}} | {{Paris/Menu}} | ||
| - | {{Paris/Header|Method & Algorithm : ƒ | + | {{Paris/Header|Method & Algorithm : ƒ8}} |
| - | [[Image:f6DCA.png|thumb|Specific Plasmid Characterisation for ƒ | + | [[Image:f6DCA.png|thumb|Specific Plasmid Characterisation for ƒ8]] |
We have <span style="color:#0000FF;">[''FlhDC'']<sub>''real''</sub> = {coef<sub>''flhDC''</sub>} ƒ1([aTc]<sub>i</sub>)</span> | We have <span style="color:#0000FF;">[''FlhDC'']<sub>''real''</sub> = {coef<sub>''flhDC''</sub>} ƒ1([aTc]<sub>i</sub>)</span> | ||
| Line 13: | Line 13: | ||
So, at steady-states, | So, at steady-states, | ||
| - | [[Image: | + | [[Image:F8.jpg|center]] |
<br> | <br> | ||
<div style="text-align: center"> | <div style="text-align: center"> | ||
| - | {{Paris/Toggle|Table|Team:Paris/Modeling/ | + | {{Paris/Toggle|Table|Team:Paris/Modeling/More_f8_Table}} |
</div> | </div> | ||
| Line 28: | Line 28: | ||
Then, if we have time, we want to verify the expected relation | Then, if we have time, we want to verify the expected relation | ||
| - | [[Image: | + | [[Image:SumpFlhB.jpg|center]] |
<br> | <br> | ||
Revision as of 14:24, 29 October 2008
|
Method & Algorithm : ƒ8
We have [FlhDC]real = {coefflhDC} ƒ1([aTc]i) and [FliA]real = {coeffliA} ƒ2([arab]i) but we use [aTc]i = Inv_ƒ1( [FlhDC] ) and [arab]i = Inv_ƒ2( [FliA] ) So, at steady-states,
↓ Table ↑
↓ Algorithm ↑
Then, if we have time, we want to verify the expected relation
<Back - to "Implementation" | |
 "
"