Team:UC Berkeley/ProjectOverview
From 2008.igem.org
(→1.2: Promoters) |
(→2.2: Dicholic Acid to Remove Outer Membrane) |
||
| Line 100: | Line 100: | ||
{Pbad}{rbs}{prepro>}{<part>}{<phoA!}{term}. The {rbs}{prepro>} composite part will have the following varients: {rbs}{prepro>}, {rbs~}{a~prepro>}, and {rbs.prepro>}. Note that we will already have the {<phoA!}{term} from the testing of individual prepro parts. The parts that we want to test in this system are: xis, int, ihfA, ihfB, Cre, ligase, BamHI, BglII. See if bugs turn yellow(?) when you induce Pbad. Similar to prepro testing experiments. We may need a {GS linker} between the part and PhoA to have a higher chance of correct folding occurring. | {Pbad}{rbs}{prepro>}{<part>}{<phoA!}{term}. The {rbs}{prepro>} composite part will have the following varients: {rbs}{prepro>}, {rbs~}{a~prepro>}, and {rbs.prepro>}. Note that we will already have the {<phoA!}{term} from the testing of individual prepro parts. The parts that we want to test in this system are: xis, int, ihfA, ihfB, Cre, ligase, BamHI, BglII. See if bugs turn yellow(?) when you induce Pbad. Similar to prepro testing experiments. We may need a {GS linker} between the part and PhoA to have a higher chance of correct folding occurring. | ||
| - | ===== '''2.2: | + | ===== '''2.2: Deoxycholic Acid to Remove Outer Membrane''' ===== |
<pre> | <pre> | ||
{pBad}{rbs}{prepro}{part}{term} | {pBad}{rbs}{prepro}{part}{term} | ||
{pBad}{rbs}{prepro}{part}{S-tag}{term} | {pBad}{rbs}{prepro}{part}{S-tag}{term} | ||
</pre> | </pre> | ||
| - | If the PhoA experiment to test protein transport to the periplasm gives a negative, that does not mean the protein was not transported - the protein may have had side reactions with the PhoA protein. At that point, we will split the parts we are trying to assay into two groups. BamHI, BglII, ligase, and cre can be linking directly to the prepro and assayed for after dissolving the outer membrane, giving parts of the format {pBad}{rbs}{prepro}{part}{term}. xis, int, ihfA, and ihfB will be linked with an S-tag we will by tagging the protein with the S-tag, giving {pBad}{rbs}{prepro}{part}{S-tag}{term}. After inducing our bacteria with arabinose, we remove the outer membrane with | + | If the PhoA experiment to test protein transport to the periplasm gives a negative, that does not mean the protein was not transported - the protein may have had side reactions with the PhoA protein. At that point, we will split the parts we are trying to assay into two groups. BamHI, BglII, ligase, and cre can be linking directly to the prepro and assayed for after dissolving the outer membrane, giving parts of the format {pBad}{rbs}{prepro}{part}{term}. xis, int, ihfA, and ihfB will be linked with an S-tag we will by tagging the protein with the S-tag, giving {pBad}{rbs}{prepro}{part}{S-tag}{term}. After inducing our bacteria with arabinose, we remove the outer membrane with deoxycholic acid, and then assay for actvity of the S-tag. |
=== Part 3 === | === Part 3 === | ||
== Results == | == Results == | ||
Revision as of 19:46, 19 June 2008
Contents |
| You can write a background of your team here. Give us a background of your team, the members, etc. Or tell us more about something of your choosing. | |
|
Tell us more about your project. Give us background. Use this is the abstract of your project. Be descriptive but concise (1-2 paragraphs) | |
| Team Example 2 |
| Home | The Team | The Project | Parts Submitted to the Registry | Modeling | Notebook |
|---|
(Or you can choose different headings. But you must have a team page, a project page, and a notebook page.)
Overall project
Our project is centered around a system that will allow sound-induced lysis o E. coli.
Project Details
Part 2
The Experiments
1: Testing Individual Composite Parts
1.1: Prepro Parts
{promoter}{rbs}{prepro}{phoA}{term}
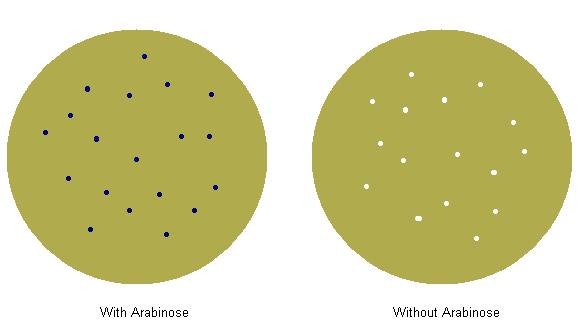
We made parts of the format {promoter}{rbs}{prepro}{phoA}{term}. The promoter we used was {pBad}, and we tried 3 different ribosome binding sites with each prepro. We plated the bacteria we transformed on two plates, both with p-nitrophenyl phosphate, but one had arabinose and one did not. We screened successful rbs/prepro combinations by looking for combinations which caused blue colonies on the plate with arabinose and white colonies on the plate without arabinose. In preparation for later composite parts, we made sure that the {rbs}{prepro} part was an intermediate when making our construction tree.
1.2: Promoters
1.2.1 Growth-dependent Promoter
{promoter}{rbs}{GFP}{term}
intermediate: {rbs}{GFP}{term}
Make parts of the format {promoter}{rbs}{GFP}{term}, where we want to make the last three, {rbs}{GFP}{term}, into one part and test the different OD dependent promoters: hns, spv, bolA, ftsAZ, ftsQ, rrnB P1, and Ptet (as a positive control, which we already have). As a negative control, we will have no promoter.
The experiment involves diluting saturated cultures and growing them at 37 C, take out at the different time points and test the fluorescence to determine at what OD they start to turn on.
1.2.2 Sound-dependent Promoter
{Psound}{rbs}{GFP}{term}
Make parts of the format {Psound}{rbs}{GFP}{term}. Grow in culture and apply sound for 30 min. Then measure fluorescence.
1.3: Amplifier
{Pbad}{spvR}{Pspv2}{rbs}{GFP}{term}
{Pbad}{rbs}{GFP}{term}
intermediates: {spvR}{Pspv2}
{rbs}{GFP}{term}
Make parts of the format {Pbad}{spvR}{Pspv2}{rbs}{GFP}{term}, where {spvR}{Pspv2} is a composite part, and {rbs}{GFP}{term} is a composite part. For the control, we will have {Pbad}{rbs}{GFP}{term}. We will grow the culture to mid-log, induce w/0.2x arabinose and measure the fluorescence after 1 hour. From this, we will determine how many folds the signal was amplified.
1.4: Ligase
{Ptet}{rbs}{ligase}{term}
{Ptet}{rbs.ligase}{term}
{Ptet}{ligase!}{term}
Make parts of the format {Ptet}{rbs}{ligase}{term}, where {Ptet}{rbs} is a composite part(which we already have). We will also make {Ptet}{rbs.ligase} and {Ptet}{ligase!}. Over-express ligase, lyse cell, and use 1 ul of cell lysate to ligate 2 purified DNA fragments.
1.5: Lysozyme, holin, antiholin
{promoter}{part}{S-tag}{term}
Assay for expression of lysozyme, holin, and antiholin. We will want to make measurements of expression levels to collect data for the modeling component, so we will want to use a variety of promoters. Assay will be done using the S-tag.
2: Testing if Protein can be Transported to the Periplasm
2.1: PhoA
{Pbad}{rbs}{prepro>}{<part>}{<phoA!}{term}
{Pbad}{rbs}{prepro>}{<part>}{<phoA!}{term}. The {rbs}{prepro>} composite part will have the following varients: {rbs}{prepro>}, {rbs~}{a~prepro>}, and {rbs.prepro>}. Note that we will already have the {<phoA!}{term} from the testing of individual prepro parts. The parts that we want to test in this system are: xis, int, ihfA, ihfB, Cre, ligase, BamHI, BglII. See if bugs turn yellow(?) when you induce Pbad. Similar to prepro testing experiments. We may need a {GS linker} between the part and PhoA to have a higher chance of correct folding occurring.
2.2: Deoxycholic Acid to Remove Outer Membrane
{pBad}{rbs}{prepro}{part}{term}
{pBad}{rbs}{prepro}{part}{S-tag}{term}
If the PhoA experiment to test protein transport to the periplasm gives a negative, that does not mean the protein was not transported - the protein may have had side reactions with the PhoA protein. At that point, we will split the parts we are trying to assay into two groups. BamHI, BglII, ligase, and cre can be linking directly to the prepro and assayed for after dissolving the outer membrane, giving parts of the format {pBad}{rbs}{prepro}{part}{term}. xis, int, ihfA, and ihfB will be linked with an S-tag we will by tagging the protein with the S-tag, giving {pBad}{rbs}{prepro}{part}{S-tag}{term}. After inducing our bacteria with arabinose, we remove the outer membrane with deoxycholic acid, and then assay for actvity of the S-tag.
 "
"