Team:University of Sheffield /Modelling
From 2008.igem.org
| Introduction | Our project | Modelling | Wet Lab | Our team | Timetable | Miscellaneous |
|---|
Mathematical Modelling
Contents |
Introduction
A mathematical model for the BarA/ UvrY and fluorescence of GFP two-component system is presented and it's dynamic behaviour is analysed. The BarA / UvrY regulates the expression of the GFP in E-coil. The model is validated in a two steps:
- The signal transduction
- The gene expression.
To survive, bacteria monitors their environment constantly and adapt to changing conditions immediately and bacteria have an established signal transduction systems to execute adaptive response to changing environmental conditions. The signal transduction system contains of two protein components: a sensor kinase anchored in the cytoplasmic membrane, and a cytoplasmic response regulator that mediates an adaptive response, that is the gene expression.
Sensory Kinase
Sensory kinases typically contain an N-terminal input domain which is connected via a linker to a C-terminal transmitter domain.
Response Regulator
Response regulators typically consist of a N-terminal receiver domain coupled to C-terminal output domains.
Upon perception of a stimulus, the input domain of the sensor kinase modulates the signalling activity of its transmitter domain, resulting in autophosphorylation with -phosphoryl group of ATP. Then, the phosphoryl group is transferred to the response regulator receiver domain, resulting in activation of the output domain(s) to trigger gene expression.
File:Doc.pdf
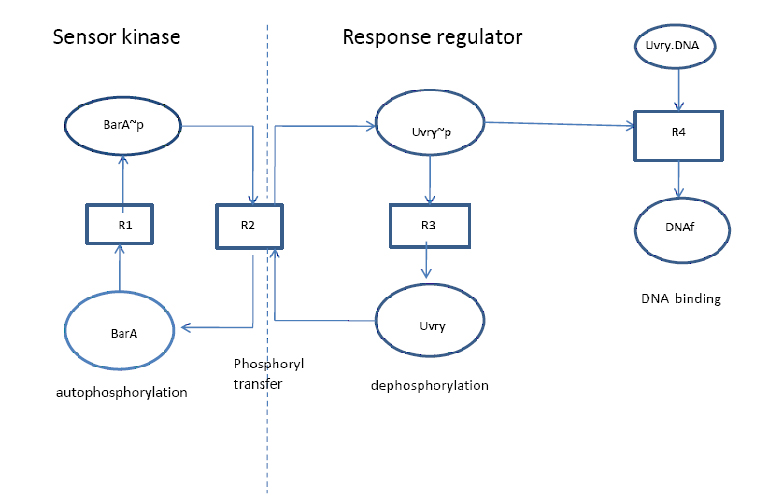
The mathematical description of a reaction mechanism representing a two component systems is presented here. The signal transduction of BarA/ Uvry expresses some reactions for auto-phosphorylation of BarA, transference of phosphoryl group of ATP between histine kinase and response regulator, and de-phosphorylation of UvrY, which is a transcription activator that initiates a response by modulating gene transcription, resulting in changes in cell physiological and metabolism to cope with the external environment.
The gene expression of CsrB and GFP are lumped together and represented by binding of the response regulator to free DNA fragments.
The Model
The model was set up describing autophosphorylation of BarA transfer of the phosphoryl group between sensor kinase and response regulator, dephosphorylation of Uvry~p, and binding of the response regulator to DNA fragments comprising the specific response regulator-binding site. The strategy to set-up and to analyse the mathematical model was constructed in vitro and validated by set of experiments.
Autophosphorylation (R1):
Phosphoryl group transfer (R2):
Dephosphorylation (R3):
DNA binding (R4):
- In reaction R1, the stimulus enhances the kinase activity that results in auto-phosphorylation of sensor kinase (BarA, BarA~p state variable) by ATP
- In reaction R2 the phosphoryl group is transferred to the response regulator (Uvry, Uvry~p state variable). Uvry~p contains the active output domain.
- In reaction R3 describes the dephosphorylation of Uvry~p by cognate sensor kinase BarA.
- In reaction R4 the activated response regulator forms a dimer and is then binds to the free DNA (DNAf, state variable) to build a transcription complex (Uvry-DNA, state variable), in presence of RNA polymerase.
Based on the analysis of the mathematical model for the two-component system a reduced model comprising O.D.Es was developed. The reduced model consist of two sub-model 1 describes the signal transduction to activate the response regulator while sub model 2 describes the interaction of the activated response regulator with DNA control sequence. The sensor kinase and the response regulator are auto-controlled. The dynamics strongly depends on the initial conditions.
For the total concentration of the sensory kinase BarA and of the response regulator UvrY, and the entire concentration of DNA fragment. The following equations hold:
 "
"