Team:University of Sheffield /Project
From 2008.igem.org


|

|

|

|

|

|
Project Overview
Contents |
Introduction
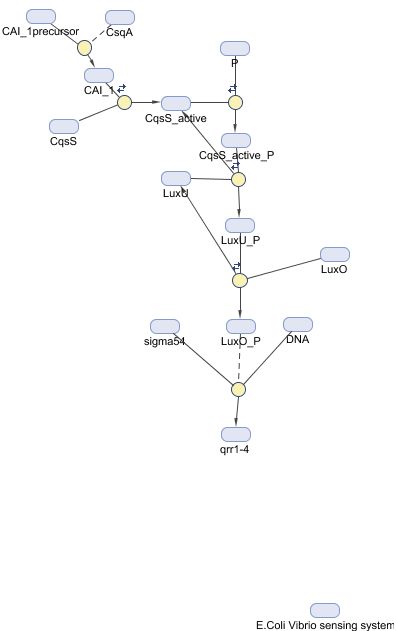
We want is to engineer a biological machine that will sense microbial contamination of drinking water using quorum sensing, namely the system in Vibrio cholerae, the bacterial species that causes epidemics of cholera. Quorum sensing systems use sensing proteins in the membrane (histidine-kinases in this case) to detect molecules excreted by the bacteria’s own species. After detection in the membrane, a phosphate signal is passed down pathway, causing a DNA regulatory effect. Each quorum-sensing species also has its own corresponding quorum-producing protein. In Vibrio cholerae the sensing protein is CqsS and the quorum-producing protein CqsA.
Initial Design Ideas
* Bacteria which digest toxins * Sensors which emit smell as an output to sensing substances * Fractal patterns and bacteria
Development of Initial Ideas
Goals
The (relatively ambitious) aim was initially to engineer a biological machine that will sense microbial contamination of drinking water using quorum sensing. The organism would initially be designed to sense a wide range of pathogens – the coliform bacterias, Escherichia coli, Shigella, Campylobacter, Salmonella enteritidis, Helicobacter sp., Vibrio cholera, Mycobacterium avium and the protozoans Giardia lamblia, Cryptosporidium sp., Cyclospora sp. and Toxoplasma sp. These cause a range of serious diseases such as dysentery, typhoid, cholera and many gastrointestinal infections.
Possible Methods
Two organisms would be modified for quorum sensing purposes, Escherichia coli and Bacillus subtilis. They represent gram negative and gram positive bacteria respectively. The choice of the class of bacteria to be used by the team will be based on suitability for expression of particular genes. The genes encoding receptor would be transferred from the target pathogenic organism to the sensing bacterium. As a result sensing bacterium would express this receptor and be able to bind the signaling molecule produced by the target organism. Subsequently, this binding would in turn trigger designated pathways within the sensing bacterium. These would lead to expression of Green Fluorescent Protein. Therefore, when pathogen is present in water, the sensing bacteria would start to fluoresce.
The design of biological pathways and circuits is going to be achieved applying mathematical tools. The intention is to invent a universal chassis with an easily exchangeable sensor part for different pathogens. It should enable straightforward switch of any intended gene and construction of various sensing biological machines on the basis of the Sheffield team design. Moreover, this way by standardizing new sets of genes the team would contribute to the Registry of Standard Biological Parts.
Implementation Overview
We are hijacking a pathway already present in E.coli – the non-essential BarA, pathway which is partly responsible for 'last resort' carbon metabolism. This pathway starts with similar histidine-kinase KdpD to Vibrio cholerae, and makes a compatible candidate topologically. We're taking the sending part of the Vibrio cholerae histidine kinase (called CqsS) and attaching it to the phosphotransfer part of the BarA histidine kinase, forming a new, fusion kinase. This is the simple way of putting it, as fusion proteins are notoriously difficult to get to work in reality. However the similiar topology between BarA and CqsS helps us somewhat, and sessions with Prof Rice (a protein crystollogropher in the Molecular Biology and Biotechnology department here) have increased our chances of a succesfulm protein. When triggered, out fusion kinase will pass the signal down E.coli's natural BarA response system to its target genes, with which we are fusing GFP. In short, is cholera autoinducers are in the water, our cell will glow! The original BarA will be knocked out of E.coli to make way for recombinant receptors (we dont want native BarA triggering our GFP!).
The recombinant protein will be expressed on a high-copy plasmid, so we should be able to get large-scale production of the recombinant proteins which may lower the threshold number of quorum molecules required to trigger the pathway.
 "
"