September
From 2008.igem.org
m |
|||
| (12 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
Content= | Content= | ||
<div style="font-size:18pt;"> | <div style="font-size:18pt;"> | ||
| - | <font face="Arial Rounded MT Bold" style="color:#010369"> | + | <font face="Arial Rounded MT Bold" style="color:#010369">_september</font></div> |
<br> | <br> | ||
<br> | <br> | ||
__NOTOC__ | __NOTOC__ | ||
| - | <h3> | + | <h3>Sep. 10th 2008</h3> |
<br> | <br> | ||
'''CMV PCR''' (Sabine)<br> | '''CMV PCR''' (Sabine)<br> | ||
| Line 31: | Line 31: | ||
No product was received. | No product was received. | ||
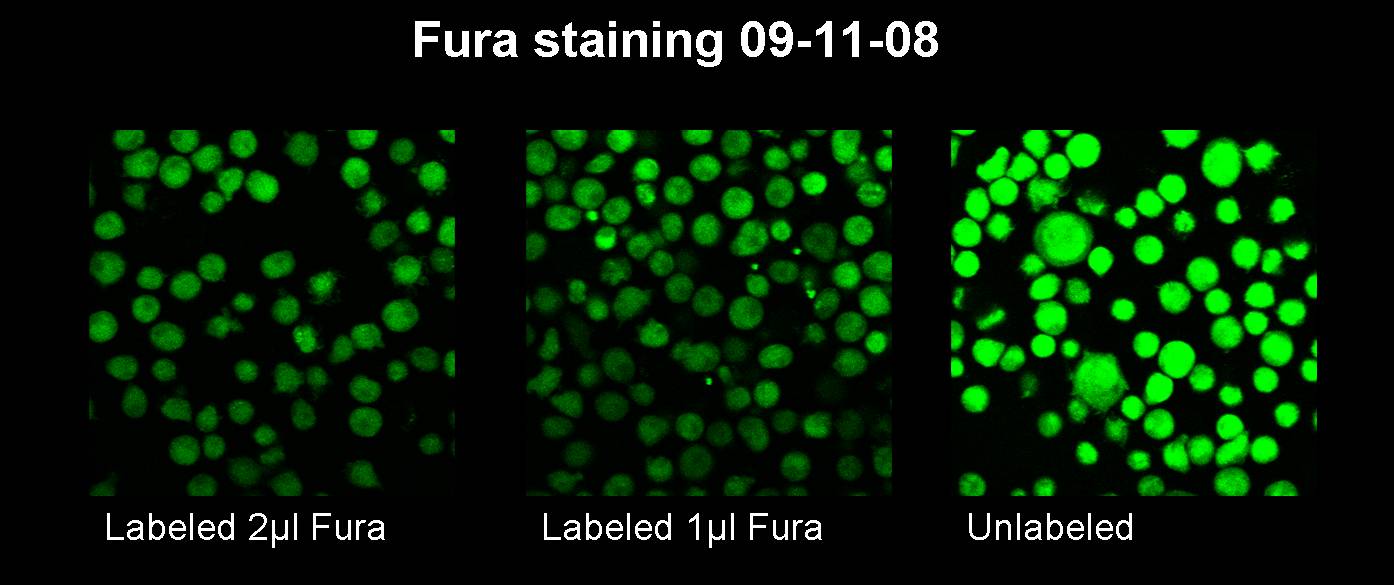
| - | <h3> | + | <h3>Sep. 11th 2008</h3> |
| - | [[image:11.09.08 Test Fura staining-Freigem08.jpg | | + | [[image:11.09.08 Test Fura staining-Freigem08.jpg |600px]]<br> |
<br> | <br> | ||
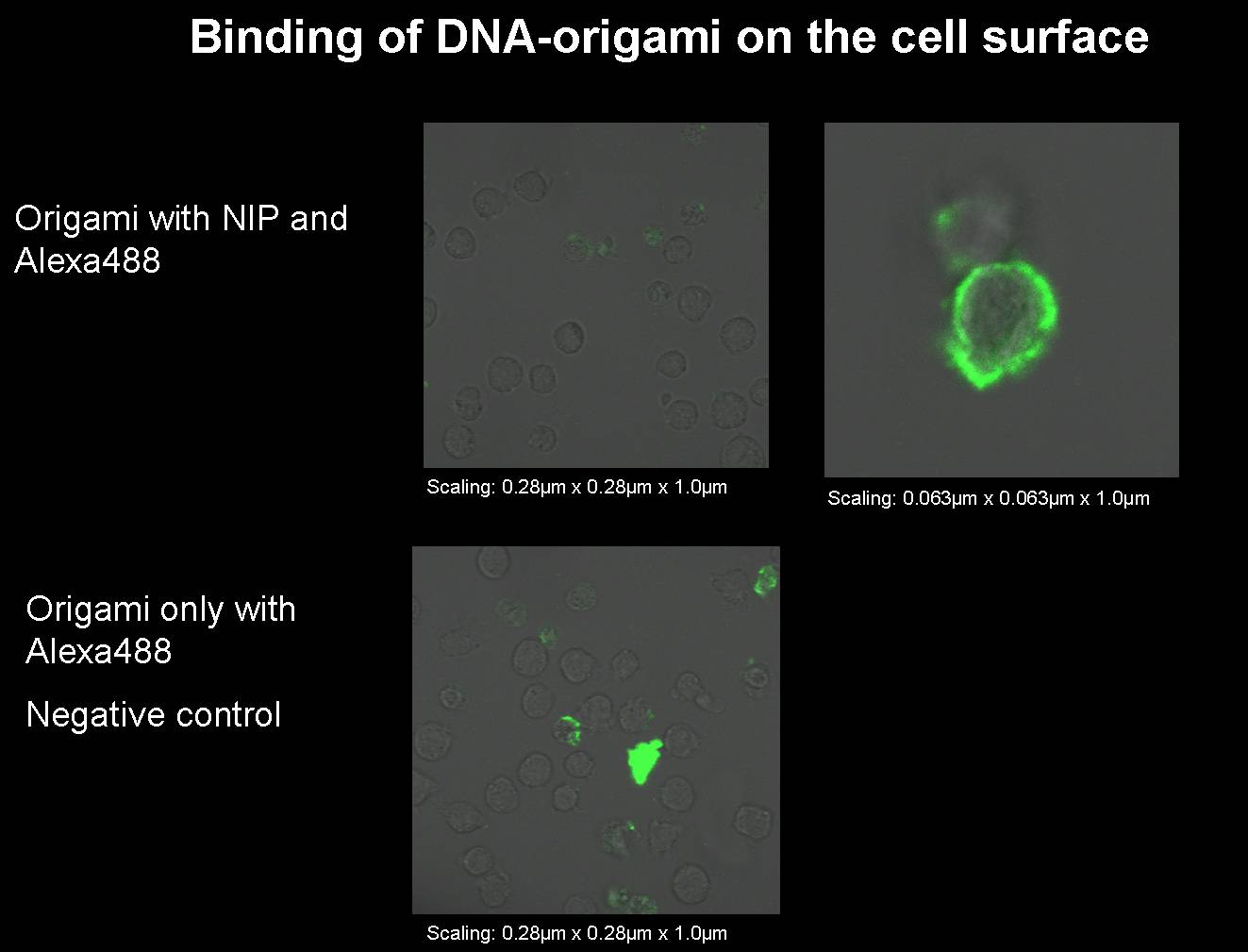
| - | [[image:11.09.08 Test Bindung Origami to alexa-Freigem08.jpg | | + | [[image:11.09.08 Test Bindung Origami to alexa-Freigem08.jpg |600px]]<br> |
| - | <h3> | + | <h3>Sep. 12th 2008</h3> |
| - | '''1) Origami with NIP and fluorophor for the binding measurement''' | + | '''1) Origami with NIP and fluorophor for the binding measurement '''(Norman+Simone)<br> |
We had to produce some new origami for our next binding measurements. | We had to produce some new origami for our next binding measurements. | ||
| Line 51: | Line 51: | ||
see at the protocol from 07-24-2008 | see at the protocol from 07-24-2008 | ||
| - | '''2) Origami for the Calciummeasurement''' | + | '''2) Origami for the Calciummeasurement '''(Norman+Simone)<br> |
* Origami with NIP | * Origami with NIP | ||
| Line 108: | Line 108: | ||
</table> | </table> | ||
| - | '''3) Master cycler''' | + | '''3) Master cycler '''(Norman+Simone)<br> |
The origamis were produced in the mastercycler as explained before. | The origamis were produced in the mastercycler as explained before. | ||
| - | '''4) Purification of the DNA Origami''' | + | '''4) Purification of the DNA Origami '''(Norman+Simone)<br> |
Was done as before | Was done as before | ||
| Line 130: | Line 130: | ||
'''6) CMV PCR''' (Sabine)<br> | '''6) CMV PCR''' (Sabine)<br> | ||
-the same PCR with different annealing temperatures using a gradient between 58°C and 62°C (optimal annealing temperature: 62°C)<br> | -the same PCR with different annealing temperatures using a gradient between 58°C and 62°C (optimal annealing temperature: 62°C)<br> | ||
| - | -again, no products were gained | + | -again, no products were gained<br><br> |
| + | |||
| + | '''7) preparation of the 293T cells for a Mg2+ and TAE-tolerancetest (MTT-assay)''' Normann | ||
| + | <ul> <li> scraping cells off the dish and centrifuging them at 1300rpm (5min) | ||
| + | <li> discarding the supernatant and washing the cells in 10ml PBS | ||
| + | <li> spinning down again at 1300rpm (5min), discarding the supernatant and solving the cells in 10ml fresh medium (DMEM) | ||
| + | <li> giving 500µl of the cellsuspension to each well of a 6 well plate and adding Medium and 125mM MgAc | ||
| + | </ul> | ||
| + | <table style="text-align: left; width: 100px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td>Well</td> | ||
| + | <td>Medium added [µl]</td> | ||
| + | <td>MgAc (125mM) added [µl]</td> | ||
| + | <td>final conentration of Mg2+[mM]</td> | ||
| + | <td>TA added ( for 30min at 16.9.08)</td> | ||
| + | |||
| + | </tr> | ||
| + | <tr><td>1</td> | ||
| + | <td>4500</td> <td>0</td> | ||
| + | <td>0</td> | ||
| + | <td>No</td> | ||
| + | </tr> | ||
| + | <tr><td>2</td> <td>4500</td> <td>0</td> | ||
| + | <td>0</td> | ||
| + | <td>Yes</td> | ||
| + | </tr> | ||
| + | <tr><td>3</td> <td>4000</td> <td>500</td> | ||
| + | <td>12,5</td> | ||
| + | <td>Yes</td> | ||
| + | </tr> | ||
| + | <tr><td>4</td> <td>4250</td> <td>250</td> | ||
| + | <td>6,25</td> | ||
| + | <td>Yes</td> | ||
| + | </tr> | ||
| + | <tr><td>5</td> <td>4250</td> <td>250</td> | ||
| + | <td>6,25</td> | ||
| + | <td>No</td> | ||
| + | </tr> | ||
| + | <tr><td>6</td> <td>4000</td> <td>500</td> | ||
| + | <td>12,5</td> | ||
| + | <td>No</td> | ||
| + | </tr> | ||
| + | </table> | ||
| - | <h3> | + | <h3>Sep. 15th 2008</h3> |
'''CMV-PCR''' (Sabine)<br> | '''CMV-PCR''' (Sabine)<br> | ||
-another effort to gain the CMV-Promotor via PCR, this time with different polymerases: Taq Polymerase and a Mix.<br> | -another effort to gain the CMV-Promotor via PCR, this time with different polymerases: Taq Polymerase and a Mix.<br> | ||
| Line 139: | Line 182: | ||
-products in the approach with taq polymerase as well as in the approach with the Mix. 3 bands from each approach were cut out | -products in the approach with taq polymerase as well as in the approach with the Mix. 3 bands from each approach were cut out | ||
| - | <h3> | + | |
| + | <h3>Sep. 16th 2008</h3> | ||
'''Gel purification''' (Sabine)<br> | '''Gel purification''' (Sabine)<br> | ||
-gel purification of the PCR products | -gel purification of the PCR products | ||
| Line 147: | Line 191: | ||
-ligation | -ligation | ||
| - | <h3> | + | |
| + | <h3>Sep. 18th 2008</h3> | ||
'''Transformation''' (Sabine)<br> | '''Transformation''' (Sabine)<br> | ||
-transformation with the ligation product. | -transformation with the ligation product. | ||
| - | <h3> | + | |
| + | <h3>Sep. 19th 2008</h3> | ||
'''Transformation''' (Sabine)<br> | '''Transformation''' (Sabine)<br> | ||
-no colonies on the plates. | -no colonies on the plates. | ||
| - | <h3> | + | |
| + | <h3>Sep. 20th 2008</h3> | ||
'''Digestion of the PCR products and the transfection-vector''' (Sabine)<br> | '''Digestion of the PCR products and the transfection-vector''' (Sabine)<br> | ||
-the restriction-enzymes XbaI and SpeI were used | -the restriction-enzymes XbaI and SpeI were used | ||
| Line 162: | Line 209: | ||
-digestion of PCR products and vector | -digestion of PCR products and vector | ||
| - | <h3> | + | |
| + | <h3>Sep. 21st 2008</h3> | ||
'''Transformation of the ligation''' (Sabine)<br> | '''Transformation of the ligation''' (Sabine)<br> | ||
-RV 308 cells were transformed with 10 µl of the ligation | -RV 308 cells were transformed with 10 µl of the ligation | ||
| + | |||
| + | <h3>Sep. 23rd 2008 </h3> | ||
| + | '''1. Transformation of the 4 plasmids of ATG''' (michael)<br> | ||
| + | -> Signalpeptide<br> | ||
| + | -> Transmembrane domain<br> | ||
| + | -> His-tag<br> | ||
| + | -> Strep-tag<br><br> | ||
| + | '''2. Digestion...''' | ||
| + | |||
| + | <h3>Sep. 24th 2008</h3> | ||
| + | '''1. Transformation ''' (michael)<br> | ||
| + | of the Ligations: CMV-Promotor + Vector<br> | ||
| + | proportion: PCR-product (CMV promoter) / vector<br> | ||
| + | -> 6/2<br> | ||
| + | -> 4/4<br><br> | ||
| + | '''2. Picking clons of the '4 ATG trafos'''' (michael)<br> | ||
| + | -> Signalpeptide<br> | ||
| + | -> Transmembrane domain<br> | ||
| + | -> His-tag<br> | ||
| + | -> Strep-tag<br> | ||
| + | after growing glycerinstocks and Miniprep of all 4<br><br> | ||
| + | '''3. Picking clons from stock''' (michael) | ||
| + | -> lipocalin<br> | ||
| + | -> C-GFP and N-GFP<br> | ||
| + | -> C-CFP and N-CFP<br> | ||
| + | -> scFv anti NIP<br><br> | ||
| + | '''4. Digestion of the CMV-promoter'''<br> | ||
| + | Approach Mix: 3/4.1/4.2/4.3/4.4<br><br> | ||
| + | ...<br> | ||
| + | <h3>Sep. 25th 2008</h3><br> | ||
| + | '''Miniprep''' (normann)<br> of C-CFP and scFv-anti-NIP<br><br> | ||
| + | '''Picking from stocks''' (normann):<br> | ||
| + | N-CFP, N-GFP, C-CFP, C-GFP<br><br> | ||
| + | '''Defrost B12-cells''' (michael)<br> | ||
| + | and put in <u>new RPMI:</u><br> | ||
| + | ->500ml RPMI<br> | ||
| + | +50ml FCS(10%)<br> | ||
| + | +5ml Hepes(1M)<br> | ||
| + | +5ml L-glutamine(200mM)<br> | ||
| + | +5ml Pen-Strep<br> | ||
| + | +1,75µl ß-Mercaptoethanol(14.3M)<br><br> | ||
| + | '''Ligation 2nd try''' (sabine)<br> | ||
| + | with T4-ligase:<br> | ||
| + | Insert: CMV-Promotor, old and new PCR-Product<br> | ||
| + | Vector: Transfection-Vector fermented with EcoRI/SpeI<br> | ||
| + | -Approaches (for each, old and new PCR-Product): Insert/Vector -> a) 6/2, b) 4/4<br><br> | ||
| + | |||
| + | <h3>Sep. 26th 2008</h3><br> | ||
| + | '''Transformation of the 'ligation 2nd try'''' (michael)<br><br> | ||
| + | '''Picking clones'''<br> | ||
| + | 4x Signalpeptide<br> | ||
| + | 2x Transmembraneregion<br><br> | ||
| + | '''Miniprep of:''' (norman)<br> | ||
| + | -Lipocalin<br> | ||
| + | -Venus split N-GFP<br> | ||
| + | -Venus split C-GFP<br> | ||
| + | -Cerulan split N-CFP<br> | ||
| + | |||
| + | <h3>Sep. 30th 2008</h3><br> | ||
| + | '''Maesurement of calcium influx<br>'''(Simone) | ||
| + | Cell staining<br> | ||
| + | 1)wash cells in medium (RPMI) with 1% FCS<br> | ||
| + | 2)count the cells and then resuspend the cells in RPMI with 1%FCS -> concentration 10^6 – 5x 10^6 cells/ml!<br> | ||
| + | 3)Mix:<br> | ||
| + | 25µl Indo-1 (5µg/ml)<br> | ||
| + | 25µl Pluronic F127 (0,5µg/ml)<br> | ||
| + | 113µl FCS<br> | ||
| + | -> mix it very well and incubate it for 5minutes<br> | ||
| + | 4)Add 15µl of the mixture/ml of cells -> incubate it in the dark for 45minutes at 37°C, shake it every 15minutes<br> | ||
| + | 5)Add 5-10ml medium (use the same medium as the one which will be used for the measurement) and centrifuge it at 1200rpm for 5minutes<br> | ||
| + | 6)Take supernatant out. And resuspend the cells to 2-5x10^6 cells/ml<br> | ||
| + | <br> | ||
| + | Measurement on the FACS<br> | ||
| + | Ca2+ response was induced by addition of the indicated stimulus 1 min after starting to record the ratio of Ca2+-bound Indo-1 versus unbound Indo-1 with a LSRII fluorescence spectrometer (Becton Dickinson). Data were analyzed with the FloJo 6.1 software.<br> | ||
| + | For the measurement: prewarm 50ml of the medium for the measurement up to 37°C, to make sure that you have the optimal conditions for the cells.<br> | ||
| + | <br> | ||
| + | Results<br> | ||
| + | [[Image:Image-Freiburg2008_FACS_result.jpg|600px]] | ||
}} | }} | ||
Latest revision as of 02:41, 30 October 2008
|
_september
Sep. 10th 2008
For a 50 µl reaction: The settings for the PCR machine are the following: No product was received. Sep. 11th 2008
Sep. 12th 20081) Origami with NIP and fluorophor for the binding measurement (Norman+Simone) We had to produce some new origami for our next binding measurements.
see at the protocol from 07-24-2008 2) Origami for the Calciummeasurement (Norman+Simone)
see at the protocol from 07-24-2008 To increase the concentration of origami we also made to probes with the double amount ingredients of the protocol from 07.24.2008
3) Master cycler (Norman+Simone) The origamis were produced in the mastercycler as explained before. 4) Purification of the DNA Origami (Norman+Simone) Was done as before 5) Digestion of CMV+Rluc (Sabine) Digestion with EcoRV und FspI (3h at 37°C)
File:Verdau CMVRluc EcoRV FspI klein.jpg
7) preparation of the 293T cells for a Mg2+ and TAE-tolerancetest (MTT-assay) Normann
Sep. 15th 2008CMV-PCR (Sabine)
Sep. 16th 2008Gel purification (Sabine) Digestion of the PCR products and the transfectionvector (Sabine, Kathrin)
Sep. 18th 2008Transformation (Sabine)
Sep. 19th 2008Transformation (Sabine)
Sep. 20th 2008Digestion of the PCR products and the transfection-vector (Sabine) Gel purification and ligation (Sabine)
Sep. 21st 2008Transformation of the ligation (Sabine) Sep. 23rd 20081. Transformation of the 4 plasmids of ATG (michael) Sep. 24th 20081. Transformation (michael) Sep. 25th 2008Miniprep (normann) Sep. 26th 2008Transformation of the 'ligation 2nd try' (michael) Sep. 30th 2008Maesurement of calcium influx |
 "
"