Team:BrownTwo/Implementation/testing
From 2008.igem.org
(→Circuit Testing) |
(→Outputs) |
||
| (One intermediate revision not shown) | |||
| Line 24: | Line 24: | ||
Our proof-of-principle design incorporates the use of inducible promoters that respond to varied levels of chemical input. | Our proof-of-principle design incorporates the use of inducible promoters that respond to varied levels of chemical input. | ||
| - | pMET25 is a | + | pMET25 is a repressable promoter that responds to levels of methionine from 0-500 uM. |
| - | + | pGAL1 is an inducible promoter that responds to the concentration of galactose between 0 and 3% | |
| - | + | ||
| - | + | ||
| + | pGAL1 normally has an all-or-nothing response, and is thus not tunable. | ||
| + | Christina Smolke of Caltech published a paper investigating the dynamics of the Gal induction system, in which she discovered that knockout of the Gal2 gene makes pGAL1 tunable, giving it a linear response to concentration of galactose. | ||
| + | Gene knockout in yeast is a surprisingly simple procedure. Amplifying the kanamycin resistance gene using primers each containing 30-40bp of homology to a site on the yeast chromosome yields a product that, when transformed into yeast, disrupts the gene at the chosen locus while integrating the Kan gene. This renders knockout cells resistant to the antibiotic G-418, which is then used for selection. We have preformed the Gal2 knockout using the primers designed for the paper, and hope to use pGAL1 as a tunable promoter | ||
==Outputs== | ==Outputs== | ||
| Line 35: | Line 36: | ||
*mCherry | *mCherry | ||
*YFP | *YFP | ||
| + | |||
| + | The spectra of these three fluorescent proteins can be separated to quantify their individual concentrations. We have had success imaging our strains on a confocal microscope, where different lasers are used to excite each fluorescent protein at a precise wavelength sequentially, minimizing the possibility of bleed-through. | ||
=Viability Tests= | =Viability Tests= | ||
Latest revision as of 04:27, 30 October 2008
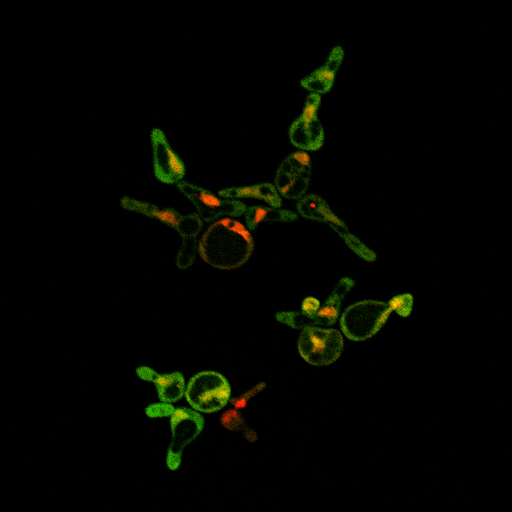
Circuit TestingWe have determined that it is necessary to characterize our parts in order to guide the construction of our device. Precisely because our system is so complex, we need to ensure that we understand the correct parts in our smaller circuits before moving on to the system as a whole. In such a case, we have outlined a few critical tests to demonstrate the proper functioning of our parts. We ran a test of our LexA activator and inducible reporter on the confocal fluorescence microscope, and saw mCherry in the nucleus and YFP in the cytoplasm as expected:
The mCherry is tagged onto the LexA activating transcription factor, and so is localized to the nucleus due to the SV40 NLS ligated just before the terminator. The YFP is generated by mCYC+LexA binding sites on a construct containing Kozak+YFP+ADH1 terminator. This strain contains the following parts: BBa_K165068 pGAL1+ LexA activator BBa_K165078 LexA activator reporter (LexA binding sites+ mCYC1+Kozak+YFPx2+ADH1 Terminator) It was grown in media containing 2% galactose and 2% raffinose. Raffinose is used as a neutral sugar that provides a carbon source while neither inducing nor repressing the pGAL1 promoter.
InputsOur proof-of-principle design incorporates the use of inducible promoters that respond to varied levels of chemical input. pMET25 is a repressable promoter that responds to levels of methionine from 0-500 uM. pGAL1 is an inducible promoter that responds to the concentration of galactose between 0 and 3% pGAL1 normally has an all-or-nothing response, and is thus not tunable. Christina Smolke of Caltech published a paper investigating the dynamics of the Gal induction system, in which she discovered that knockout of the Gal2 gene makes pGAL1 tunable, giving it a linear response to concentration of galactose. Gene knockout in yeast is a surprisingly simple procedure. Amplifying the kanamycin resistance gene using primers each containing 30-40bp of homology to a site on the yeast chromosome yields a product that, when transformed into yeast, disrupts the gene at the chosen locus while integrating the Kan gene. This renders knockout cells resistant to the antibiotic G-418, which is then used for selection. We have preformed the Gal2 knockout using the primers designed for the paper, and hope to use pGAL1 as a tunable promoter Outputs
The spectra of these three fluorescent proteins can be separated to quantify their individual concentrations. We have had success imaging our strains on a confocal microscope, where different lasers are used to excite each fluorescent protein at a precise wavelength sequentially, minimizing the possibility of bleed-through. Viability TestsIn addition to testing the promoters and parts provided to us, we wish to apply our network to tests that involve Apoptosis test- induce apoptosis externally and then save cells with the limiter device! -this test has important medical relevance, seeing as many tumor phenotypes are characterized by a noted decline or absence of apoptotic activity -for yeast, extrinsically-signaled apoptosis depends on oxidative stress or salt -involves the following system: Possible target for downregulation could be Bir1p (from BIR1 gene), a homolog of survivin from humans that is thought to stabilize XIAP Another possible target is AIF1, a homolog of the human AIF1 -released from mito.and travels to nucleus -interesting fact about S. cerevisiae is that they are facultative anaerobic yeast and can survive even with complete removal of mitochondria, making them an ideal system for studying PCD (Frolich- Yeast apoptosos) http://www.nature.com/embor/journal/v6/n11/full/7400514.html |
 "
"