Team:Paris/July 24
From 2008.igem.org
(Difference between revisions)
(→Results of digestions : Electrophoresis) |
(→MiniPreps) |
||
| (2 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
== MiniPreps == | == MiniPreps == | ||
| - | *The | + | *The Promega MiniPreps protocol has been used on all the clones cultivated on the 23th. |
{| border="1" | {| border="1" | ||
| Line 40: | Line 40: | ||
| | | | ||
|} | |} | ||
| - | |||
| - | |||
== Digestion == | == Digestion == | ||
| Line 60: | Line 58: | ||
* Store on ice | * Store on ice | ||
* Revelation of the digestion by electrophoresis on agarose gel | * Revelation of the digestion by electrophoresis on agarose gel | ||
| + | |||
| + | |||
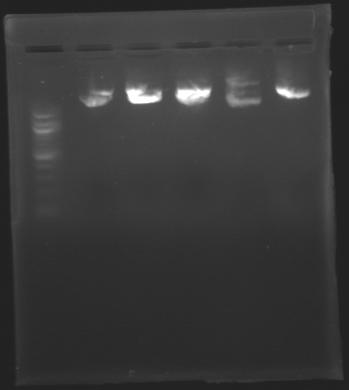
=== Results of digestions : Electrophoresis === | === Results of digestions : Electrophoresis === | ||
| Line 523: | Line 523: | ||
| - | ==> Conclusion: | + | ==> Conclusion: Most of the digestion have succeed |
== Extraction of the DNA == | == Extraction of the DNA == | ||
Latest revision as of 14:39, 31 July 2008
|
MiniPreps
DigestionDigestion Mix10µl of Miniprep (26 aug.)
Results of digestions : Electrophoresisconditions :
Extraction of the DNA
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"