Team:Bologna/Notebook
From 2008.igem.org
(→Week 6: from 08/25/08 to 08/31/08) |
(→Week 7: from 09/01/08 to 09/07/08) |
||
| Line 164: | Line 164: | ||
= Week 7: from 09/01/08 to 09/07/08 = | = Week 7: from 09/01/08 to 09/07/08 = | ||
| + | Arrival of the operator library (link) from GeneArt | ||
| + | |||
| + | *Implementation of the protocol to separate each Lac Operator from the library | ||
| + | #Single digestion with Pst and control gel run | ||
| + | In this way we open the plasmid in 3 points,leaving free the Lac Operator1 and 2, remaining the plasmid with the lac Operator 3 | ||
| + | #Gel extraction of the upper band containing Lac Operator3 inside the plasmid | ||
| + | |||
| + | #Single digestion with Xba and control gel run | ||
| + | #Gel extraction of the upper band containing Lac Operator1 inside the plasmid | ||
| + | |||
| + | #Single digestion with EcoRI and control gel run | ||
| + | In this way we open the plasmid in 2 points,leaving free the Lac Operator3, remaining the plasmid with the lac Operator1 and 2 | ||
| + | #Gel extraction of the upper band containing Lac Operator1 e Lac Operator2 inside the plasmid | ||
| + | #Further single digestion with Pst and control gel run | ||
| + | #Gel extraction of the upper band containing Lac Operator2 | ||
| + | |||
| + | This protocol was executed for both Tet, Lex, Lambda operators | ||
= Week 8: from 09/08/08 to 09/14/08 = | = Week 8: from 09/08/08 to 09/14/08 = | ||
Revision as of 16:46, 28 October 2008
| HOME | TEAM | PROJECT | MODELING | WET-LAB | SOFTWARE | SUBMITTED PARTS | BIOSAFETY AND PROTOCOLS |
|---|
Protocols summary
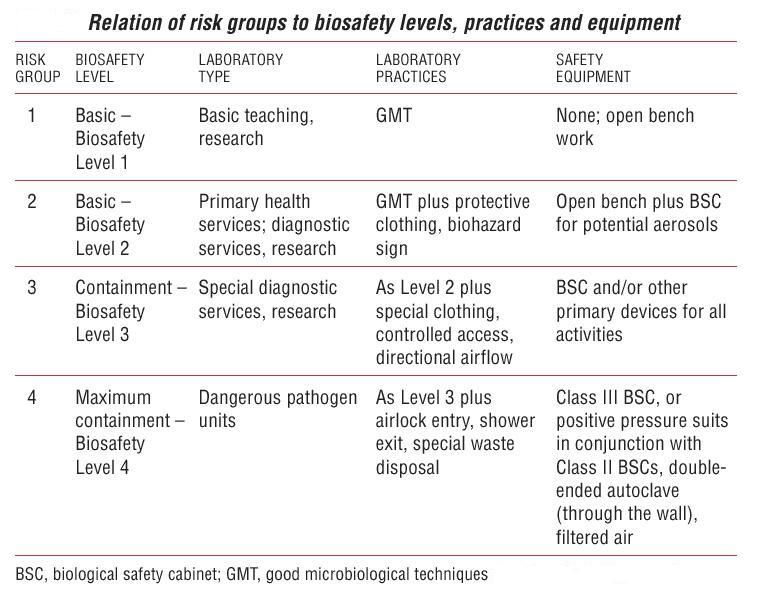
| Protocol | Biosafety level |
| Plates preparation | |
| Amplification | |
| Transformation | |
| Inoculation | |
| Miniprep | |
| Digestion reaction | |
| Gel preparation | |
| Electrophoretic run | |
| Gel extraction | |
| Ligation reaction | |
| Chemiocompetent cells | |
| Mediums and buffers | |
| Antibiotics stocks preparation | |
| IPTG stocks preparation | |
| Fluorescence test | |
| M9 supplemented media | |
Week 1: from 07/21/08 to 07/27/08
General Preparations
- Preparation of chemiocompetent cells from E. Coli DH5α, Top10 and DB 3.1
- Preparation of antibiotic stocks for Ampicillin and Kanamicin
- Preparation of LB medium and LB plates for cloning.
Week 2: from 07/28/08 to 08/03/08
- Eluition and Amplification from 2008 Registry Collection: [http://partsregistry.org/Part:BBa_R0082 R0082,] [http://partsregistry.org/Part:BBa_R0083, R0083], [http://partsregistry.org/wiki/index.php/Part:BBa_M30109 M30109] in TOP10 strain to build and characterize the Light response system to be our spatial selective trigger.
- Eluition and Amplification from 2008 Registry Collection: [http://partsregistry.org/Part:BBa_E0240 E0240], [http://partsregistry.org/Part:BBa_P1010 pSB3K3_P1010]in DB3.1 and the Practice Promoter Set ([http://partsregistry.org/wiki/index.php?title=Part:BBa_J23103/ J23150, J23151, J23102]) to test and set up the new [http://partsregistry.org/Measurement Biobrick Standard Measurement Protocol]
- Transformation and Amplification from our Lab Stock of [http://partsregistry.org/Part:BBa_S0100 S0100], BBa_I763020, [http://partsregistry.org/wiki/index.php?title=Part:BBa_I763005 I763005] and [http://partsregistry.org/Part:BBa_C0051 C0051]
- Growth Curves of Dh5 Alpha, Top10 and XL1 Blue with Low Medium and High Copy Numbers to assay and define the different kinetics (Further Detail)
Week 3: from 08/04/08 to 08/10/08
08/04/08
- Digestion and Control Gel Run of the previous amplified constructs :
1. S0100 E/S
Consistent Part Length
2. PLAC-CI X/P
Consistent Part Length
3. R0083 S/P
Single Vector Band as Expexted. Is Hard to verify the Part length correctness given the small size
4. R0082 S/P
Single Vector Band as Expexted. Is Hard to verify the Part length correctness given the small size
5. C0051 X/P
Consistent Part Length.
7. M30105 E/S
The Part appears not consistent. The Gel has unexpected multiple bands.
8. RBS GFP TAG X/P
Consistent Part Length
9.Pλ GFP X/P
Consistent Part Length.
- Ligation of R0082 and R0083 with E0240 to obtain a Reporter for the Light Driven Trigger.
Week 4: from 08/11/08 to 08/17/08
HOLIDAY
Week 5: from 08/18/08 to 08/24/08
Starting development of protein construct
- Ligation of I763020 and B0015, of S0100 and B0015, of TETR and B0015
- Trasformation of the ligations in E.coli
- Inoculation and preparation of miniprep
- Digestion and control gel run of the constructs: GFP T x\p, S0100 T x\p, TETR T x\p
- Gel extraction of the part
Week 6: from 08/25/08 to 08/31/08
- Ligation of B0034 and TetR T , B0034 and GFP T
- Trasformation in E.coli
- Inoculation and preparation of Miniprep
- Digestion and control gel run of the constructs: RBS TETR T x\p, RBS GFP T x\p
- Gel extraction of the parts
- Ligation of RBS GFP T and S0100, RBS GFP T and RBS TetR
- Trasformation in E.coli
- Inoculation and preparation of Miniprep
- Digestion and control gel run of the parts: RBS TETR RBS GFP T x\p, S0100 RBS GFP T x\p
- Gel extraction
- Termination of the constructs:
- Ligation of promotor J23118 and RBS GFP T, promotor J23105 and RBS GFP T, promotor J23100 and RBS GFP T
- Trasformation in E.coli
- Inoculation and preparation of Miniprep
- Digestion and control gel run of the constructs:J23118 RBS GFP T, J23105 RBS GFP T, J23100 RBS GFP T
- Gel extraction
Week 7: from 09/01/08 to 09/07/08
Arrival of the operator library (link) from GeneArt
- Implementation of the protocol to separate each Lac Operator from the library
- Single digestion with Pst and control gel run
In this way we open the plasmid in 3 points,leaving free the Lac Operator1 and 2, remaining the plasmid with the lac Operator 3
- Gel extraction of the upper band containing Lac Operator3 inside the plasmid
- Single digestion with Xba and control gel run
- Gel extraction of the upper band containing Lac Operator1 inside the plasmid
- Single digestion with EcoRI and control gel run
In this way we open the plasmid in 2 points,leaving free the Lac Operator3, remaining the plasmid with the lac Operator1 and 2
- Gel extraction of the upper band containing Lac Operator1 e Lac Operator2 inside the plasmid
- Further single digestion with Pst and control gel run
- Gel extraction of the upper band containing Lac Operator2
This protocol was executed for both Tet, Lex, Lambda operators
Week 8: from 09/08/08 to 09/14/08
Week 9: from 09/15/08 to 09/21/08
Week 10: from 09/22/08 to 09/28/08
Week 11: from 09/29/08 to 10/05/08
Start preparing to LEXA_2 operator reporter construct:
- first day
- digestion X/P of B0034-J04031-B0010-B0012
- digestion S/P of LEXA_2 operator
- Control Gel Run of B0034-J04031-B0010-B0012 digested X/P and LEXA_2 operator digested S/P
- Gel extraction of B0034-J04031-B0010-B0012 digested X/P and LEXA_2 operator digested S/P
- ligation of B0034-J04031-B0010-B0012 digested X/P with LEXA_2 operator digested S/P
- trasformation of LEXA_2-B0034-J04031-B0010-B0012
- second day
- inoculation of LEXA_2-B0034-J04031-B0010-B0012
- miniprep of LEXA_2-B0034-J04031-B0010-B0012
- third day
- digestion X/P of LEXA_2-B0034-J04031-B0010-B0012
- digestion S/P of J23118
- control gel run of LEXA_2-B0034-J04031-B0010-B0012 digested X/P and J23118 digested S/P
- gel extraction of LEXA_2-B0034-J04031-B0010-B0012 digested X/P and J23118 digested S/P
- ligation of LEXA_2-B0034-J04031-B0010-B0012 digested X/P with J23118 digested S/P
- trasformation of J23118-LEXA_2-B0034-J04031-B0010-B0012
- fourth day
- inoculation of J23118-LEXA_2-B0034-J04031-B0010-B0012
- miniprep of J23118-LEXA_2-B0034-J04031-B0010-B0012
- since construct test was successful, we have proceeded with the preparation of the same construct for the other two LEXA operator
Week 12: from 10/06/08 to 10/12/08
Week 13: from 10/13/08 to 10/19/08
Week 14: from 10/20/08 to 10/26/08
Week 15: from 10/27/08 to 10/29/08
 "
"


