Team:TUDelft/Temperature design
From 2008.igem.org
Bavandenberg (Talk | contribs) |
Bavandenberg (Talk | contribs) (→Design phase I: Introducing an RNA thermometer into Escherichia coli) |
||
| (37 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
{{Template:TUDelftiGEM2008_menu_home}} | {{Template:TUDelftiGEM2008_menu_home}} | ||
| - | >> | + | =Design phase I: <br /><br />Introducing an RNA thermometer into ''Escherichia coli''= |
| - | + | The design of artificial RNA thermometers is split in two phases. In the first phase, some known RNA thermometers are turned into BioBrick Standard Biological Parts, and in the [[Team:TUDelft/Temperature_design2|second phase]], RNA thermometers with different switching temperatures are designed. This page is devoted to the first design phase, in which the parts [http://partsregistry.org/wiki/index.php?title=Part:BBa_K115001 <code>BBa_K115001</code>], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K115002 <code>BBa_K115002</code>], and | |
| - | [http://partsregistry.org/wiki/index.php?title=Part: | + | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K115003 <code>BBa_K115003</code>] have been designed. |
| - | [http://partsregistry.org/wiki/index.php?title=Part: | + | |
| - | [http://partsregistry.org/wiki/index.php?title=Part: | + | |
| - | = | + | ==Three existing RNA thermometers== |
| - | The first phase | + | The goal of the first phase is to test whether known RNA thermometers can be turned into a BioBrick Standard Biological Part and incorporated into ''Escherichia coli''. We selected three RNA thermometers from literature, one per RNA thermometer family. For each of these RNA thermometers it has been shown that they can be introduced into ''E. coli'', where they still function as a temperature sensitive switch. |
| - | + | The first RNA thermometer is one of the Repressor Of heat-Shock gene Expression (ROSE) family and is retrieved from the organism ''Bradyrhizobium japonicum'' <span id="cite_ref_1">[[Team:TUDelft/Temperature_design#cite_note_1|[1]]]</span>. This RNA thermometer is switched off at 30°C and allows induction of translation by heating to 42°C. The second RNA thermometer is one of the FourU family and is retrieved from ''Salmonella''. This pathogenic microorganism expresses virulence proteins only inside a host <span id="cite_ref_2">[[Team:TUDelft/Temperature_design#cite_note_2|[2]]]</span>. An induction temperature of this switch should be around 37°C, the body temperature of the host. The third RNA thermometer is retrieved from the ''Listeria monocytogenes'' and belongs to the PrfA family. Translation is induced at 37°C <span id="cite_ref_3">[[Team:TUDelft/Temperature_design#cite_note_3|[3]]]</span>. | |
| - | == | + | ==From RNA thermometer to Standard Biological Part== |
| - | + | ||
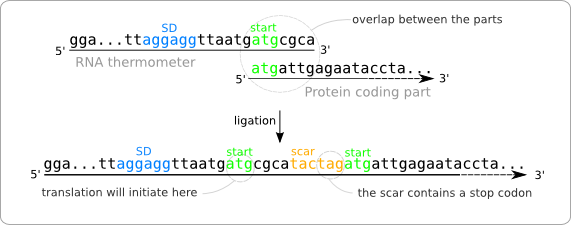
| - | + | [[Image:Overlap.png|thumb|200px|right|'''Figure 1:''' Overlap between the RNA thermometer and the protein coding part. These parts cannot be ligated this way because of the stop codon that is part of the scar.]] | |
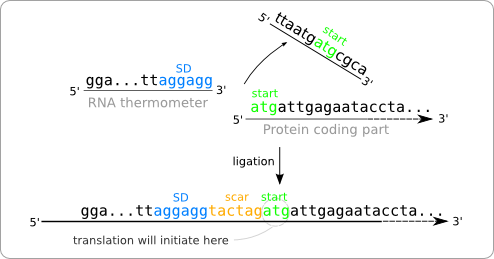
| + | [[Image:Chopped.png|thumb|200px|right|'''Figure 2:''' The part of the RNA thermometer sequence that needs to be chopped of, in order to end up with a sequence in which the Shine Dalgarno region and the start codon of the protein coding part are separated by the desired six nucleotides.]] | ||
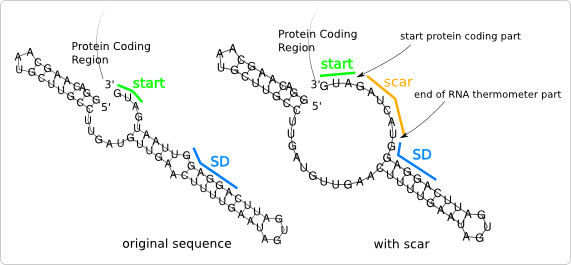
| + | [[Image:ScarProblemss.png|200px|thumb|right|'''Figure 3:''' Introduction of the scar causes a change in the secondary structure of the RNA thermometer.]] | ||
| + | [[Image:ScarProblem.png|thumb|200px|right|'''Figure 4:''' The original secondary structure is regained by alteration of the nucleotides opposite of the scar region.]] | ||
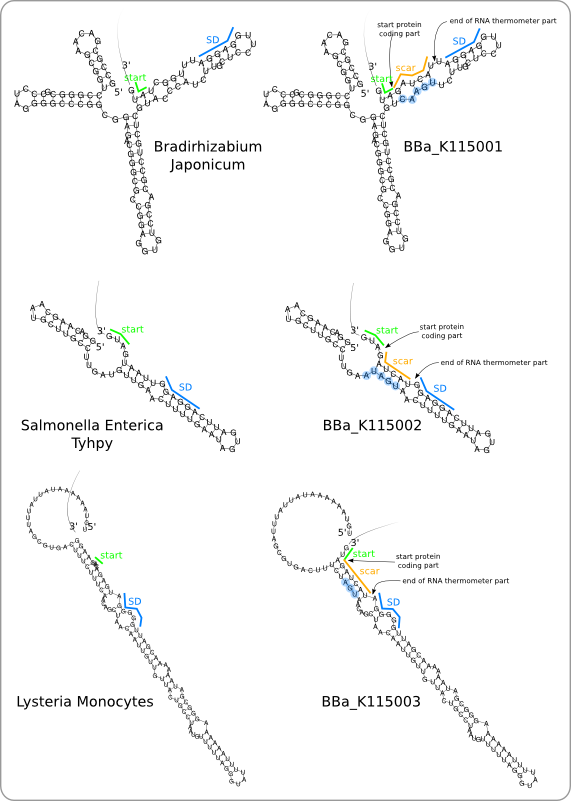
| + | [[Image:Design1.png|thumb|200px|right|'''Figure 5:''' Three RNA thermometer parts. At the left, the secondary structures of the original sequences is given. At the right, the secondary structure of the parts, as they are added to the registry, are given. The blue dots around the nucleotides indicate the mutation that had to be made in order to adjust the secondary structure after introduction of the scar.]] | ||
| - | + | Turning a protein coding gene into a Standard Biological Part is straightforward. If the DNA sequence contains any of the forbidden restriction sites, the only thing that has to be done is to remove those. Turning the RNA thermometer sequences into Standard Biological Parts is less straightforward. The reason for this is that the start codon of the protein coding part, following the RNA thermometer, is also part of the RNA thermometer sequence as found in literature ([https://2008.igem.org/Image:Overlap.png figure 1]). | |
| - | + | ====Getting the right sequence==== | |
| - | + | A resulting sequence after ligation of two parts will contain a scar in between the two ligated parts. If both the RNA thermometer sequence and the protein coding sequence are kept intact, the resulting sequence after ligation will be the one shown at the bottom of [https://2008.igem.org/Image:Overlap.png figure 1]. The resulting sequence contains two start codons of which only the leftmost one will act as initiation point for the translation. This is because of its distance to the Shine Dalgarno sequence, which should be six or seven nucleotides. | |
| - | + | ||
| - | + | ||
| - | + | Unfortunately there is a problem with this resulting sequence. Because of the shift of the start codon to the 5' side, some extra amino acids will be added to the protein. This could already by a problem, but even worse, the scar contains a stop codon. This will cause the translation process to be terminated even before the protein coding region is reached. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | Another option would be to chop off the 3' end side of the RNA thermometer sequence. In order to get a gap of six nucleotides between the SD and the start codon, the RNA thermometer sequence has to be chopped of after the SD sequence as shown in [https://2008.igem.org/Image:Chopped.png figure 2]. The resulting sequence after ligation contains a SD and a start codon separated by six nucleotides, the same as the distance between them in the original sequence of the RNA thermometer. | |
| - | + | ====Getting the right secondary structure==== | |
| - | [[Image: | + | But a new problem arises when we look at the [[Team:TUDelft/Temperature_analysis#RNA_secondary_structure_and_free_energy|secondary structure]] of this new sequence. As can be seen in [https://2008.igem.org/Image:ScarProblemss.png figure 3], the secondary structure of the new sequence (after ligation to a protein coding part) is different compared to the secondary structure of the original sequence of the RNA thermometer, while this secondary structure is essential for the temperature sensitivity. |
| - | + | Through the change of the nucleotides in the scar region, the base pairing with the opposite base pairs is lost. The original secondary structure can be regained by changing the nucleotides opposite the scar region, so that the nucleotides in that region form base pairs again ([https://2008.igem.org/Image:ScarProblem.png figure 4]). | |
| - | + | ==Result== | |
| - | + | The example in the previous section has shown how the FourU RNA thermometer sequence that is retrieved from ''Salmonella'' can be turned into a Standard Biological Part ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K115002 <code>BBa_K115002</code>]). The same method has also been used for the design of the other two RNA thermometer parts that are based on sequences from ''Bradyrhizobium japonicum'' and ''Listeria monocytogenes''. This has resulted in the two new parts: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K115001 <code>BBa_K115001</code>] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K115003 <code>BBa_K115003</code>] respectively. The secondary structures of the original sequences and of the designed sequences (after ligation to a protein coding part) are shown in [https://2008.igem.org/Image:Design1.png figure 5]. | |
| - | + | An alternative solution would be to combine the RNA thermometer sequence and the protein coding sequence into one part, instead of having a separate part for both sequences. The downside of this solution is that a new part has to be added to the registry for each protein that needs to be regulated by temperature. Conforming the iGEM vision it is desired to make a separate temperature sensitive part that can be reused in combination with any protein coding part without going over the same design process over and over again. That is why separate temperature sensitive parts have been designed. | |
| - | + | ==References== | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<ol class="references"> | <ol class="references"> | ||
| - | <li id=" | + | <li id="cite_note_1"> [[Team:TUDelft/Temperature_design#cite_ref_1 | ^]] Saheli Chowdhury, Christophe Maris, Frédéric H-T Allain, and Franz Narberhaus. Molecular basis for temperature sensing by an RNA thermometer. ''The EMBO Journal'', 25:2487–2497, 2006. [http://www.ncbi.nlm.nih.gov/pubmed/16710302 PMID:16710302] </li> |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | <li id=" | + | <li id="cite_note_2"> [[Team:TUDelft/Temperature_design#cite_ref_2 | ^]] Torsten Waldminghaus, Nadja Heidrich, Sabine Brantl, and Franz Narberhaus. FourU: a novel type of RNA thermometer in Salmonella. ''Molecular Microbiology'', 65(2):413-424, 2007. [http://www.ncbi.nlm.nih.gov/pubmed/17630972 PMID:17630972]</li> |
| - | <li id=" | + | <li id="cite_note_3"> [[Team:TUDelft/Temperature_design#cite_ref_3 | ^]] J . Johansson, P . Mandin, A . Renzoni, C . Chiaruttini, M . Springer, and P . Cossart. An RNA thermosensor controls expression of virulance genes in Listeria monocytogenes. ''Cell'' , 110(5):551-561, 2002. [http://www.ncbi.nlm.nih.gov/pubmed/12230973 PMID:12230973] </li> |
</ol> | </ol> | ||
{{Template:TUDelftiGEM2008_sidebar}} | {{Template:TUDelftiGEM2008_sidebar}} | ||
Latest revision as of 07:59, 29 October 2008
Contents |
Design phase I:
Introducing an RNA thermometer into Escherichia coli
The design of artificial RNA thermometers is split in two phases. In the first phase, some known RNA thermometers are turned into BioBrick Standard Biological Parts, and in the second phase, RNA thermometers with different switching temperatures are designed. This page is devoted to the first design phase, in which the parts [http://partsregistry.org/wiki/index.php?title=Part:BBa_K115001 BBa_K115001], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K115002 BBa_K115002], and
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K115003 BBa_K115003] have been designed.
Three existing RNA thermometers
The goal of the first phase is to test whether known RNA thermometers can be turned into a BioBrick Standard Biological Part and incorporated into Escherichia coli. We selected three RNA thermometers from literature, one per RNA thermometer family. For each of these RNA thermometers it has been shown that they can be introduced into E. coli, where they still function as a temperature sensitive switch.
The first RNA thermometer is one of the Repressor Of heat-Shock gene Expression (ROSE) family and is retrieved from the organism Bradyrhizobium japonicum [1]. This RNA thermometer is switched off at 30°C and allows induction of translation by heating to 42°C. The second RNA thermometer is one of the FourU family and is retrieved from Salmonella. This pathogenic microorganism expresses virulence proteins only inside a host [2]. An induction temperature of this switch should be around 37°C, the body temperature of the host. The third RNA thermometer is retrieved from the Listeria monocytogenes and belongs to the PrfA family. Translation is induced at 37°C [3].
From RNA thermometer to Standard Biological Part
Turning a protein coding gene into a Standard Biological Part is straightforward. If the DNA sequence contains any of the forbidden restriction sites, the only thing that has to be done is to remove those. Turning the RNA thermometer sequences into Standard Biological Parts is less straightforward. The reason for this is that the start codon of the protein coding part, following the RNA thermometer, is also part of the RNA thermometer sequence as found in literature (figure 1).
Getting the right sequence
A resulting sequence after ligation of two parts will contain a scar in between the two ligated parts. If both the RNA thermometer sequence and the protein coding sequence are kept intact, the resulting sequence after ligation will be the one shown at the bottom of figure 1. The resulting sequence contains two start codons of which only the leftmost one will act as initiation point for the translation. This is because of its distance to the Shine Dalgarno sequence, which should be six or seven nucleotides.
Unfortunately there is a problem with this resulting sequence. Because of the shift of the start codon to the 5' side, some extra amino acids will be added to the protein. This could already by a problem, but even worse, the scar contains a stop codon. This will cause the translation process to be terminated even before the protein coding region is reached.
Another option would be to chop off the 3' end side of the RNA thermometer sequence. In order to get a gap of six nucleotides between the SD and the start codon, the RNA thermometer sequence has to be chopped of after the SD sequence as shown in figure 2. The resulting sequence after ligation contains a SD and a start codon separated by six nucleotides, the same as the distance between them in the original sequence of the RNA thermometer.
Getting the right secondary structure
But a new problem arises when we look at the secondary structure of this new sequence. As can be seen in figure 3, the secondary structure of the new sequence (after ligation to a protein coding part) is different compared to the secondary structure of the original sequence of the RNA thermometer, while this secondary structure is essential for the temperature sensitivity.
Through the change of the nucleotides in the scar region, the base pairing with the opposite base pairs is lost. The original secondary structure can be regained by changing the nucleotides opposite the scar region, so that the nucleotides in that region form base pairs again (figure 4).
Result
The example in the previous section has shown how the FourU RNA thermometer sequence that is retrieved from Salmonella can be turned into a Standard Biological Part ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K115002 BBa_K115002]). The same method has also been used for the design of the other two RNA thermometer parts that are based on sequences from Bradyrhizobium japonicum and Listeria monocytogenes. This has resulted in the two new parts: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K115001 BBa_K115001] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K115003 BBa_K115003] respectively. The secondary structures of the original sequences and of the designed sequences (after ligation to a protein coding part) are shown in figure 5.
An alternative solution would be to combine the RNA thermometer sequence and the protein coding sequence into one part, instead of having a separate part for both sequences. The downside of this solution is that a new part has to be added to the registry for each protein that needs to be regulated by temperature. Conforming the iGEM vision it is desired to make a separate temperature sensitive part that can be reused in combination with any protein coding part without going over the same design process over and over again. That is why separate temperature sensitive parts have been designed.
References
- ^ Saheli Chowdhury, Christophe Maris, Frédéric H-T Allain, and Franz Narberhaus. Molecular basis for temperature sensing by an RNA thermometer. The EMBO Journal, 25:2487–2497, 2006. [http://www.ncbi.nlm.nih.gov/pubmed/16710302 PMID:16710302]
- ^ Torsten Waldminghaus, Nadja Heidrich, Sabine Brantl, and Franz Narberhaus. FourU: a novel type of RNA thermometer in Salmonella. Molecular Microbiology, 65(2):413-424, 2007. [http://www.ncbi.nlm.nih.gov/pubmed/17630972 PMID:17630972]
- ^ J . Johansson, P . Mandin, A . Renzoni, C . Chiaruttini, M . Springer, and P . Cossart. An RNA thermosensor controls expression of virulance genes in Listeria monocytogenes. Cell , 110(5):551-561, 2002. [http://www.ncbi.nlm.nih.gov/pubmed/12230973 PMID:12230973]
 "
"