Team:BrownTwo/Implementation/syntf
From 2008.igem.org
| Line 1: | Line 1: | ||
{{limitertemp}} | {{limitertemp}} | ||
| - | |||
| + | =The Biofusion Standard= | ||
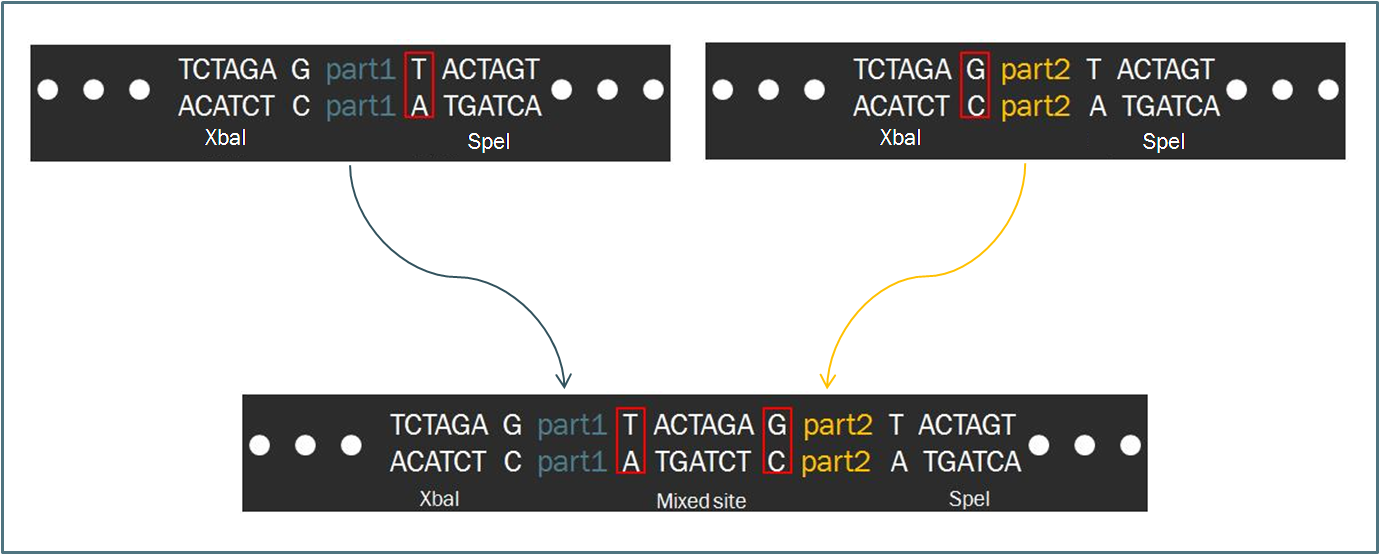
| - | The | + | Compared to other fields in biology, synthetic biology devotes a considerable amount of attention towards the standardization of parts and of practice. The inspiration for such a focus stems from similar concepts in engineering, which depends upon well-characterized components for the design of complex systems that behave reliably according to predictions. Indeed, as readers of this wiki may be well aware, the iGEM competition focuses on the use of the Biobrick standard, which allows for an idempotent approach to cloning recombinant DNA. The Biobrick standard constitutes a conserved sequence of four restriction enzyme cut sites that envelop a genetic part, whether it be a coding gene, a promoter, or some other functional piece of DNA. Using the process of Standard Assembly, one can then ligate two parts together by following a formulaic scheme of , with the end result being a ligation of two genetic parts together while maintaining the same found. |
| - | + | As a slight deviation from this system, our synthetic transcription factors were designed according to a Biofusion standard, which was developed by the Silver lab at Harvard for the purpose of constructing these parts. | |
| - | + | ||
| - | The | + | The differences between the two standards are minimal. In fact, the method of ligating two parts proves to be exactly the same, regardless of whether the part is destined to become a prefix or a suffix to another. Such a result is a consequence of the fact that the two standards share an identical layout of restriction digest sites. However, a close-up investigation of the interface that occurs when two parts from each scheme are ligated together. |
| - | + | As one can see in the diagram, intrinsic to the Biobrick standard is a spacing nucleotide between the part and each of the adjacent restriction sites, namely XbaI and SpeI. When two such parts are ligated together according to the Standard Assembly procedure, exactly one spacer nucleotide from each Biobrick is maintained in the final part. The result is that a total of eight nucleotides lie between , comprised of these two spacers plus the hybrid | |
| - | + | [[image:Biobrickin'.jpg|thumb|center|900px]] | |
| - | + | [[image:Biofusion.jpg|center|900px]] | |
| - | |||
| - | + | By nature of how it is designed, the Biobrick standard affords a great deal of modularity to those who use parts . Thus, the Biofusion provides for an even more customizable system by allowing for the creation of fusion proteins, hybrid proteins formed by the fusion of two protein domains to one another. This modularity for protein design lies at the heart of our limiter device. | |
| - | |||
| + | =Transcription factors = | ||
| - | + | The syn. trans. factor system designed by caroline & david. cite memory. | |
| + | |||
| + | *modularity | ||
| + | *extensibility | ||
| + | |||
| + | The laboratory of Dr. Pamela Silver at Harvard developed synthetic transcription factors for the construction of a novel “memory device” in Saccharomyces cerevisiae. While the details of this device are not essential to understanding our own gene network, it is important to discuss the significance of these transcription factors to our design. A transcription factor is composed of a binding domain, which targets the protein either an activation domain or a repression domain, which | ||
| + | |||
| + | Transcription factors are composed of a standard activation or repression domain linked to a variable binding domain. The binding domain is chosen such that it matches. Other features of these . A handful of these parts were available in the Registry, but we found it necessary to | ||
| - | |||
| - | |||
Modularity – in order to modify the design to limit the expression of a given gene of interest, one must study a gene pathway map and identify a single link between the gene of interest and a transcription factor that regulates the expression of that gene | Modularity – in order to modify the design to limit the expression of a given gene of interest, one must study a gene pathway map and identify a single link between the gene of interest and a transcription factor that regulates the expression of that gene | ||
Revision as of 18:06, 29 October 2008
|
The Biofusion StandardCompared to other fields in biology, synthetic biology devotes a considerable amount of attention towards the standardization of parts and of practice. The inspiration for such a focus stems from similar concepts in engineering, which depends upon well-characterized components for the design of complex systems that behave reliably according to predictions. Indeed, as readers of this wiki may be well aware, the iGEM competition focuses on the use of the Biobrick standard, which allows for an idempotent approach to cloning recombinant DNA. The Biobrick standard constitutes a conserved sequence of four restriction enzyme cut sites that envelop a genetic part, whether it be a coding gene, a promoter, or some other functional piece of DNA. Using the process of Standard Assembly, one can then ligate two parts together by following a formulaic scheme of , with the end result being a ligation of two genetic parts together while maintaining the same found. As a slight deviation from this system, our synthetic transcription factors were designed according to a Biofusion standard, which was developed by the Silver lab at Harvard for the purpose of constructing these parts. The differences between the two standards are minimal. In fact, the method of ligating two parts proves to be exactly the same, regardless of whether the part is destined to become a prefix or a suffix to another. Such a result is a consequence of the fact that the two standards share an identical layout of restriction digest sites. However, a close-up investigation of the interface that occurs when two parts from each scheme are ligated together. As one can see in the diagram, intrinsic to the Biobrick standard is a spacing nucleotide between the part and each of the adjacent restriction sites, namely XbaI and SpeI. When two such parts are ligated together according to the Standard Assembly procedure, exactly one spacer nucleotide from each Biobrick is maintained in the final part. The result is that a total of eight nucleotides lie between , comprised of these two spacers plus the hybrid
Transcription factorsThe syn. trans. factor system designed by caroline & david. cite memory.
The laboratory of Dr. Pamela Silver at Harvard developed synthetic transcription factors for the construction of a novel “memory device” in Saccharomyces cerevisiae. While the details of this device are not essential to understanding our own gene network, it is important to discuss the significance of these transcription factors to our design. A transcription factor is composed of a binding domain, which targets the protein either an activation domain or a repression domain, which Transcription factors are composed of a standard activation or repression domain linked to a variable binding domain. The binding domain is chosen such that it matches. Other features of these . A handful of these parts were available in the Registry, but we found it necessary to
Stipulation: If G is a transcription factor that is known to auto-regulate, one will have to alter the modeling to account for this situation. modifications to current scheme:
Possible further modifications to these transcription factors include the use of different regulation domains, Involves the consideration of multiple binding sites |
 "
"