Team:BrownTwo/Implementation/syntf
From 2008.igem.org
| Line 53: | Line 53: | ||
Other parts of these synthetic transcription factors include | Other parts of these synthetic transcription factors include | ||
| + | |||
| + | The inherent fluorescence of the | ||
=Other schemes of regulation= | =Other schemes of regulation= | ||
| + | |||
| + | While our device was built to function solely on the level of transcription, one can easily imagine the opportunity for other modes of regulation. Our repressor | ||
| + | |||
Revision as of 23:52, 29 October 2008
|
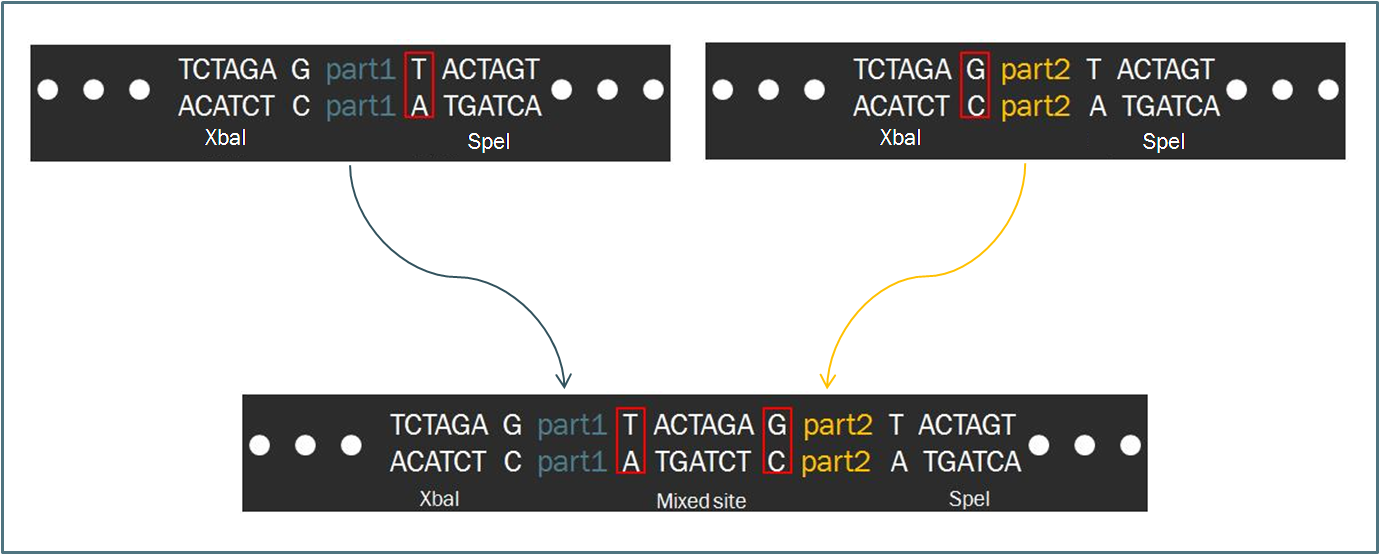
The Biofusion StandardCompared to other fields in biology, synthetic biology devotes a considerable amount of attention towards the standardization of parts and of practice. The inspiration for such a focus stems from similar concepts in engineering, which depends upon well-characterized components for the design of complex systems with predictable and reliable behavior. Indeed, as readers of this wiki may know, the iGEM competition focuses on the use of the Biobrick standard, which allows for an idempotent approach to cloning recombinant DNA. The Biobrick standard constitutes a conserved sequence of four restriction enzyme cut sites that envelop a genetic part, whether it be a coding gene, a promoter, or some other functional piece of DNA. According to the process of Standard Assembly, one can then ligate two such parts together after following a formulaic scheme of restriction digest reactions. The end result is a linking of two two genetic parts bounded by the same four restriction sites in a reproducible fashion. Such a system facilitates the pairing of two parts, allowing for the creation of endless combinations while constructing a device.
Intrinsic to the Biobrick standard is the placement of a single nucleotide between the part and each of its adjacent restriction sites, namely XbaI and SpeI. The nucleotides are intended to act as spacers to protect the part from enzyme cleavage. When two Biobrick parts are ligated together according to the Standard Assembly, exactly one spacer nucleotide from each Biobrick is maintained in the composite part. The result is a total of eight nucleotides that lie between the two parts, comprised of these two spacers plus the hybrid XbaI/SpeI site.
The difference in separation length between two DNA parts is significant with respect to translation. During protein synthesis, ribosomes recognize groups of three consecutive nucleotides known as codons and translate each codon into a particular amino acid. Should an additional nucleotide appear in the mRNA sequence, the the grouping of nucleotides into threes will be shifted, resulting in an entirely new and undesired sequence of amino acids.
Therefore, since the length of the sequence separating two Biobrick parts is not divisible by three, a frame shift occurs. If the second Biobrick happens to be a protein-coding sequence of DNA, it will not be translated correctly. Biofusion improves upon these constraints, enabling a synthetic biologist to append numerous protein-coding regions to one another.
By nature of their design, both Biobrick and Biofusion parts can be utilized in the design of modular devices. However, the Biofusion standard provides for even greater customization by allowing for the creation of fusion proteins, hybrid proteins formed by the fusion of protein domains to one another to generate a single functional protein with characteristics from its constitutive parts.
This modular scheme for protein design lies at the heart of our limiter device. Synthetic Transcription factorsExcluding the case of our proof-of-principle design, our limiter device is composed entirely of synthetic transcription factors created according to the Biofusion standard. The laboratory of Dr. Pamela Silver at Harvard developed these synthetic transcription factors for the construction of a novel “memory device” in Saccharomyces cerevisiae. While the details of this device are not essential to understanding our own gene network, it is important to discuss the significance of these transcription factors to our design.
The inherent fluorescence of the Other schemes of regulationWhile our device was built to function solely on the level of transcription, one can easily imagine the opportunity for other modes of regulation. Our repressor
Other features of these . A handful of these parts were available in the Registry, but we found it necessary to
Modularity – in order to modify the design to limit the expression of a given gene of interest, one must study a gene pathway map and identify a single link between the gene of interest and a transcription factor that regulates the expression of that gene Stipulation: If G is a transcription factor that is known to auto-regulate, one will have to alter the modeling to account for this situation. modifications to current scheme:
Possible further modifications to these transcription factors include the use of different regulation domains, Involves the consideration of multiple binding sites |
 "
"