Team:Chiba/Project/Experiments:Sender Crosstalk
From 2008.igem.org
| Home | The Team | The Project | Parts Submitted to the Registry | Reference | Notebook | Acknowledgements |
|---|
Sender Crosstalk
Design
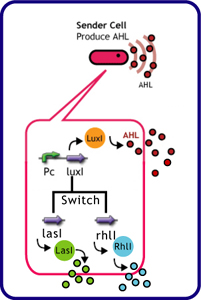
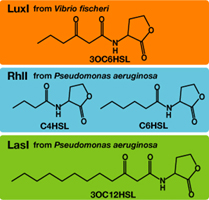
Each species has their own LuxI-type proteins,which synthesize their specific autoinducers, AHLs. AHLs produced by different LuxI-type proteins differ only in the length of the acyl-chain moiety and substitution at position C-3.LuxR,which is original for Vibrio fischeri, is activated by its cognate autoinducer, 3OC6HSL. However, LuxR is also activated by non-endogenous molecules, C4HSL, C6HSL, and 3OC12HSL. Activation by non-endogenous molecules requires a higher signal concentration (2). This results in slower activation of receivers, when AHL concentration is increasing.
Experiments
The purpose of this experiment was to create delays in communication time using cross-talk between non-specific signals. We used the following genes for this experiment.
| senders (cell: XL10-Gold or JW1908) | receiver (cell: JW1908) |
| *[http://partsregistry.org/Part:BBa_K084007 plac+rbs+LasI] (pSB1AK3) | *[http://partsregistry.org/Part:BBa_T9002 BBa_T9002 (Express GFP in response to AHL)] (pSB1A3) |
| *[http://partsregistry.org/Part:BBa_K084008 plac+rbs+RhlI] (pSB1AK3) | |
| *[http://partsregistry.org/Part:BBa_K084009 BBa_K084009] | |
| *[http://partsregistry.org/Part:BBa_K084012 plac+rbs+LuxI] (pSB1AK3) | |
| *[http://partsregistry.org/Part:BBa_K084014 BBa_K084014] | |
| *[http://partsregistry.org/Part:BBa_S03623 BBa_S03623(ptet+rbs+LuxI(LVA))] |
Details
Method
To characterize quorum sensing crosstalk, constitutive AHL senders were mixed with constitutive receivers and measure fluorescence intensity.
- Transformed Senders into E.coli strains (XL10Gold) and Receiver into E.coli strain (JW1908).
- Inoculated them independently in liquid media. Incubated at 37°C for 12hours.
- Mixed them.
- Incubated at 30°C.
- Measured intensity of green fluorescence (485 nm (excitation) and 527 nm (emission)) at regular time intervals.
Results & Discussion
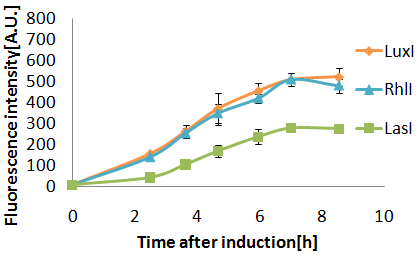
Senders(XL10Gold), T9002(JW1908)@30°C, Sender(μL):Receiver(μL)=500:500, 100:1000, 10:1000
- LasIを形質転換入した細胞は37°Cよりも、30°Cにおいて盛んにAHLを合成している(と思われる).
At 30 degrees Celsius, the E.coli(XL10G) transformed with the LasI genes composed more AHL than 37 degrees Celsius. (intensity of green fluorescence after incubating for 8 hours at 37°C:30°C=163:245)
- LasI gene(BBa_K084007)によって合成された3OC12HSLとLuxI gene(BBa_K084012)によって合成される3OC6HSLによって合成されるAHLは,ともにLuxRタンパク質を活性化した.3OC12HSLの発現による蛍光強度の上昇は,3OC6HSLのそれよりもゆるやかであり,蛍光強度200に達するまでの時間に2.5時間の差がみられた.その時の条件は,培養液体積比=1:1,培養温度30°C,AHL senderの株は,XL10Gold,AHL receiverの株はJW1908であった.
- 3OC12HSL synthesized by LasI protein and 3OC6HSL synthesized by LuxI protein both activated LuxR protein and cousese gfp expression .The increase of green fluorescence intensity caused by 3OC12HSL was more gradualy than that caused by 3OC6HSL,time before fluorescence intensity reached at 200 was 2.5 hours longer than that of the 3OC6HSL.
Experimental conditions were shown in below:
- reaction temparature was 30°C.
- The ratio of culture volumes was 1:1.
- LuxI gene was transformed with E.coli strain XL10Gold. LasI gene was transformed with E.coli strain JW1908.
- The sender genes LuxI and LasI was produce the greatest time difference, 2.5 hours.
- Rhlのタグなしとタグ付きの差は、8h後の最終強度にしか現れなかった。
- We observed the difference between Rhl and Rhl on last data which was measured 8 hours later.
- LVA-tag had effect only on the maximum fluorescence intensity induced by two signal molecules.
- また、差が一番でたのが30°Cで行ったこの実験で、タグなし:タグつき=507:456。
3OC6HSL,3OC12HSLに対する、LuxRの応答時間に、二時間の差ができた(3OC12HSLの場合、3OC6HSLに比べて二時間遅れて応答する)。 (そのときの条件は、培養温度30°C、genelatorの株はXL10Gold,Receiverの株はJW1908のとき、であった。) <??
- LasI gene worked more actively at 30°C than worked at 37°C.(Fuluorecence intensity after 8 hours incubating at 37°C was 163,on the other hand,Fuluorecence intensity after 8 hours incubating at 30° was 245.)
- The time to achieve 200 of fuluorecence intensity with LasI and LuxR (pLac)(=1:1) was delayed 2.5 hours.
- RhlI and RhlI (with LVA tag) made a difference of the latest intensities after 8 hours.
- And,this conbination (@30°C) made a greatest differences.(no LVA tag):(LVA tag)=507:456.
When we used the cross-talk strategy using 3OC12HSL instead of 3OC6HSL, the delayed switch worked 2 hours after the original switch worked. Futher variation of delayed switches will be realized:
Future plans
- Reduce quantity of expression of an enzyme composing AHL
- Replace medium RBS with weak one.
- Alter the copy number of plasmids in the sender into low.
- To make the slope of transfer curve of our device to be steep:
- Introduce Positive Feedback Loop into our gene circuit.
Other Experiments
Visual Judgement
- Fluorescence intensity was from 150 to 200 when the 2 mL of reaction culture in a test tube.
Senders(JW1908), T9002(JW1908)@37°C, Sender(μL):Receiver(μL)=500:500, 100:1000, 10:1000
- Among RhlI, LasI, and CinI+LVA, RhlI+LVA is the easist to cross-talk with LuxR
- Delay time by cross-talk among senders are generated only a range of one hour to amount
to 200 of fluorescence intensity.
Senders(JW1908),T9002(JW1908)@30°C,Sender(μL):Receiver(μL)=500:500,100:1000,10:1000
Senders(XL10Gold),T9002(JW1908)@37°C, Sender(μL):Receiver(μL)=500:500,100:1000,10:1000
Senders(XL10Gold), T9002(JW1908)@25°C, Sender(μL):Receiver(μL)=500:500, 100:1000, 10:1000
Demo ~Senders~
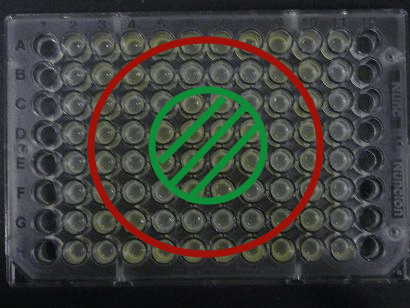
We experimentally tested the sender genes LuxI and LasI which produce the greatest time difference. We mixed bacteria (XL10G) transformed with either the LuxI or LasI genes and bacteria (of the BW strain) transformed with the LuxR gene at a 1:1 ratio and visually observed GFP fluorescence.
Results
Green region: sender=LuxI (50 uL), Red circular region: sender=Las I (50 uL).
Receiver=LuxR (50 uL)
-->more about Demo experiments detail
references
- [http://www3.interscience.wiley.com/journal/119124142/abstract M.K Winson et al.:Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing.FEMS Microbiology Letters 163 (1998) 185-192]
- [http://partsregistry.org/Part:BBa_F2620:Specificity BBa_F2620:Specificity]
- [http://mic.sgmjournals.org/cgi/content/full/153/12/3923 Paul Williams.:Quorum sensing, communication and cross-kingdom signalling in the bacterial world.Microbiology 153 (2007), 3923-3938]
 "
"