Team:Chiba/Calendar-Home/3 September 2008
From 2008.igem.org
2 September 2008 <|> 4 September 2008
Contents |
Laboratory work
Team:Input
no work
Team:Communication
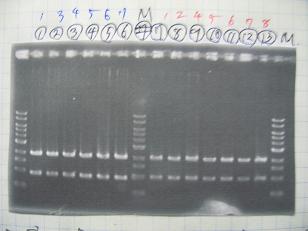
- (2/9)--->Mini prep
- [http://partsregistry.org/Part:BBa_K084009 BBa_K084009](R1, R3~R7)
- [http://partsregistry.org/Part:BBa_K084010 BBa_K084010](C1,C2,C4~C8)
- [http://partsregistry.org/Part:BBa_S03154 BBa_S03154]
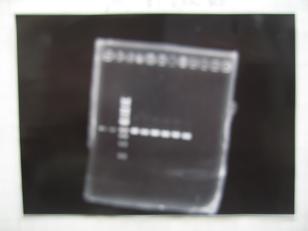
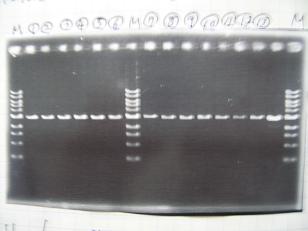
- --->Gel Check

|
|
- --->Digestion test
- [http://partsregistry.org/Part:BBa_K084009 BBa_K084009](R1, R3~R7)
- [http://partsregistry.org/Part:BBa_K084010 BBa_K084010](C1,C2,C4~C8)
| Digestion | Single | Double |
| Sample DNA(μL) | 1 | 3 |
| XbaⅠ(μL) | 0.1 | 0.1 |
| PstⅠ(μL) | 0.1 | 0.1 |
| Buffer 2(μL) | 0.9 | - |
| Buffer 3(μL) | - | -0.8 |
| BSA(μL) | 1 | 1 |
| dH2O(μL) | 7 | 5 |
| TOTAL(μL) | 10 | 10 |
- --->Gel Check
|
|
|
|
- (2/9)--->Phenotype-test
- MIX
- [http://partsregistry.org/Part:BBa_K084007 BBa_K084007](Plac+RBS+LasI, Competent Cells : JW1908) -> Sample Name : L1~L4
- [http://partsregistry.org/Part:BBa_K084008 BBa_K084008](Plac+RBS+RhlI, Competent Cells : JW1908) -> Sample Name : R1~R4
- [http://partsregistry.org/Part:BBa_S03623 BBa_S03623](Ptet+RBS+LuxI, Competent Cells : JW1908)
- [http://partsregistry.org/Part:BBa_T9002 BBa_T9002](Ptet+RBS+LuxR+GFP, Competent Cells : JW1908)
Sample No. 1 2 3 4 5 6 7 8 9 10 11 L1 2mL - - - - - - - - - - L2 - 2mL - - - - - - - - - L3 - - 2mL - - - - - - - - L4 - - - 2mL - - - - - - - R1 - - - - 1mL - - - - - - R2 - - - - - 1mL - - - - - R3 - - - - - - 1mL - - - - R4 - - - - - - - 1mL - - - [http://partsregistry.org/Part:BBa_S03154 BBa_S03154] - - - - - - - - 2mL - - AHL(100μM) - - - - - - - - - - 1μL [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] 2mL 2mL 2mL 2mL 1mL 1mL 1mL 1mL 2mL 1mL 1mL IPTG(100μM) 1μL 1μL 1μL 1μL 1μL 1μL 1μL 1μL - - - - Incubated for 8hours at 37 degrees
- Spindown (max rpm, 3 min)
- measured the intensity GFP(visual judgment)
Sample No. 1 2 3 4 5 6 7 8 9 10 11 the intensity GFP + + + + + + + ++ + +++ -
- Left for 12 at room temperture.
- Resuspensioned
- measured the intensity of GFP by Fluoroskan Ascent 2.5(program:Ascent Software Version 2.6)
Sample No. 1 2 3 4 5 6 7 8 9 10 11 the intensity GFP 25.65 18.91 20.39 21.85 26.62 26.58 30.07 33.60 27.41 10.72 55.54 Team:Output
- [http://partsregistry.org/Part:BBa_R0079 BBa_R0079](1)
- [http://partsregistry.org/Part:BBa_R0071 BBa_R0071](2)
- [http://partsregistry.org/Part:BBa_R0077 BBa_R0077](3)
- [http://partsregistry.org/Part:BBa_R0078 BBa_R0078](4)
- [http://partsregistry.org/Part:BBa_R0062 BBa_R0062](5)
Sample No. 1~5 DNA tamplate(μL) 5 Buffer3(μL) 1 dH2O(μL) 4 EcoRI(μL) 0.1 TOTAL(μL) 10 -->37°C30min
-->Gel check
Sample No. 1~5 DNA(digested sample(μL)) 10 loading Dye(μL) 2 TOTAL(μL) 12
 "
"