Team:Wisconsin/Project
From 2008.igem.org
| Home | The Team | The Project | Parts Submitted to the Registry | Modeling | Notebook |
|---|
Abstract
The global fuel crisis impacts our economy, national security, and environment. The need for alternative fuels is of utmost importance. Team Wisconsin used Escherichia coli to produce biofuel precursors in an effort to find these alternative fuel sources. One project focused on using E. coli to efficiently produce sorbitol, a biofuel precursor. Using computer modeling, we determined a way to funnel glycolysis’ intermediates towards the production of sorbitol via sorbitol-6-phosphate dehydrogenase. Wisconsin’s second aim was to isolate high-energy precursors from the plant cell wall through E. coli mediated breakdown of cell wall lignin. This was achieved by inserting the gene encoding lignin peroxidase , found in the white rot fungus Phanerochaete chrysosporium, into genetically modified E. coli, capable of producing and exporting the enzyme. Both projects improve current methods in the production of alternative fuels via two different, unique routes, and have the potential to move sustainable biofuel research forward.
Introduction
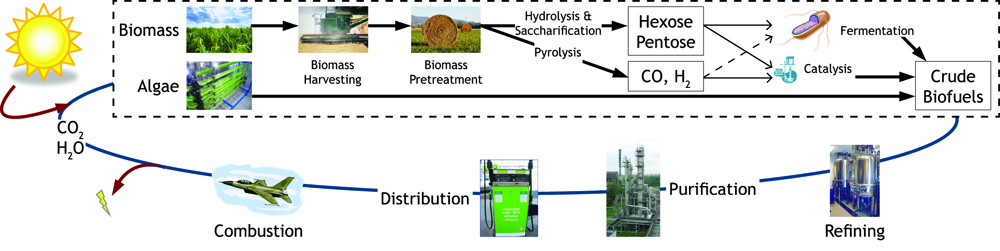
Currently in the United States the biofuel of choice is ethanol produced from corn. Only the starch within the kernel is fermented despite the whole plant being harvested. The ratio of energy input to energy output is 1:1.34, which is inefficient for a system that is being used as a fuel source. Cellulose fermentation seems the obvious next step in biofuel production since cellulose is about 33% of all plant matter and the most abundant organic compound on earth. This has been recently accomplished by several individuals and organizations with industrial production still in the works. Advances in cellulosic biofuel technology are predicted to create an energy content of up to four billion barrels of crude oil, which accounts for 65% of oil consumption in America. Government support is assisting this transition away from fossil fuels- in 2007, US Department of Energy announced that $385 million in grants will be provided to fund six different cellulosic ethanol plants. Further support from private institutions and constant scientific advances in biofuel production methods will provide better alternatives and a change that is desperately needed. Due to the increased costs of fossil fuels, it has now become imperative to harness renewed energy through biofuel synthesis.
Figure 1: Map of forms of sustainable chemical production.
Overall Project
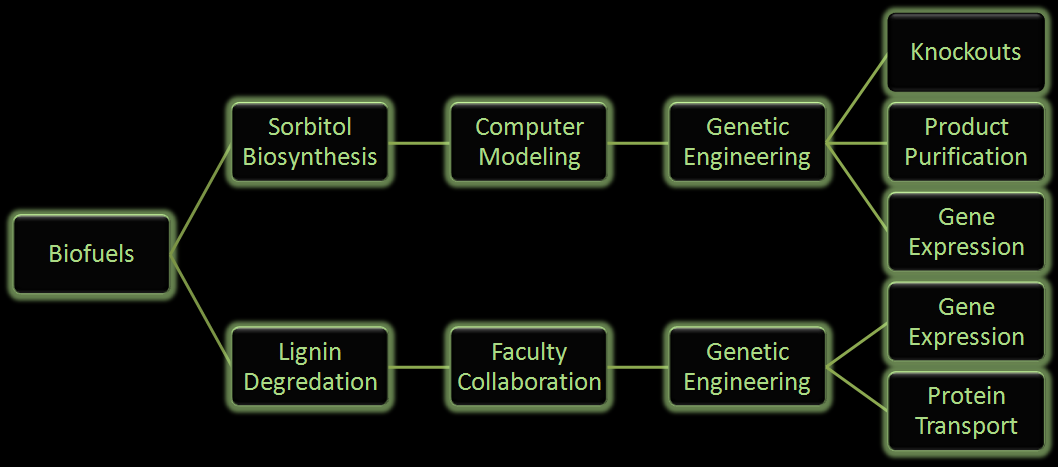
Fuel production has come to the forefront as an important political and biological issue. It has lead to innovative pursuits of renewable fuel as well as the controversial exploitation of natural resources. Currently ethanol is the commercial biofuel of choice; however, current production and distillation of ethanol in the United States is inefficient. With this problem in mind, the iGEM Wisconsin team is looking for alternative ways to make not only ethanol, but other biofuels through synthetic biology. We've designed the following two projects based around using E. coli to produce biofuels.
Figure 2: Flow chart illustrates the process our team followed in creating our genetically engineered cells.
One project focuses on using E. coli to produce sorbitol, a sugar alcohol, in large quantities for eventual commercial scale catalytic conversion to hydrocarbons. Along with producing sorbitol, we've modeled alterations in E. coli to make sorbitol production from a glycerol carbon source possible. Our aim is to modify a cell that will utilize glycerol, a byproduct from biodiesel production, and effectively create sorbitol.
In the other project we are attempting to use E. coli to break down lignin from plant matter. This would increase the efficiency of biofuel production from plant biomass. We are currently aiming to insert a fungal gene coding for lignin peroxidase into E. coli. Lignin breakdown will be made possible through the transport of lignin peroxidase out of the cell.
Lignin Peroxidase
Lignin is the second most abundant organic compound on earth. It is a very complex, polymorphic phenyl-propane polymer. Lignin is considered one of the hardest organic polymers to degrade due to its insolubility, high molecular weight, non-linear and non-uniform structure. Only about 10% of lignin is phenolic, meaning that ether linkages must be cleaved to break it down.
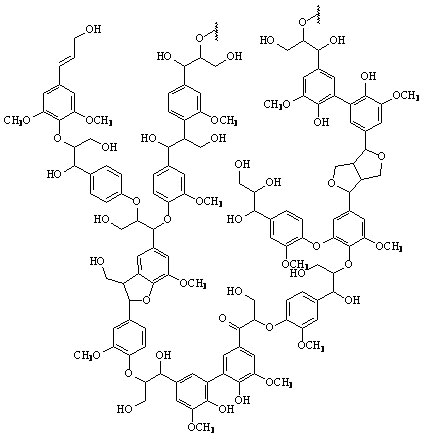
Figure 3: A proposed structure of lignin. (Taken from: [http://www.research.uky.edu/odyssey/winter07/green_energy.html The University of Kentucky])
Lignin, cellulose and hemi-cellulose are the three main components of the cell well. Lignin fills the space between cellulose and hemi-cellulose covalently bonding repeatedly to both. This hinders cell wall breakdown and in nature prevents access to cellulose as a substrate for enzymes.
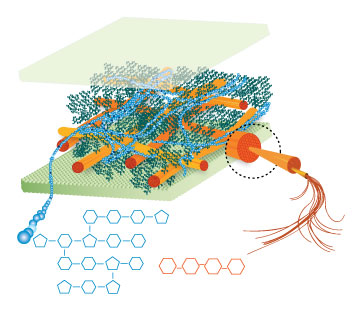
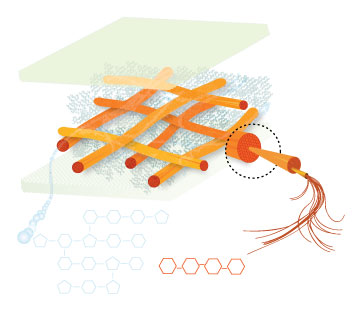
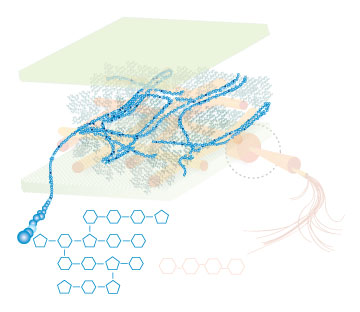
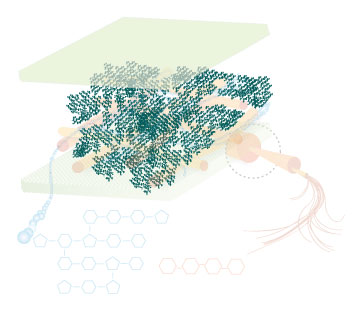
Figure 4: These images show the different components of the plant cell wall complex: cellulose(orange), hemi-cellulose(blue), and lignin(green). (Taken from: [http://www.ceres.net/AboutUs/AboutUs-Biofuels-Carbo.html Ceres: the energy crop company])
There are enzymes and organisms out there that can break down lignin. Adding genes necessary for the enzymatic breakdown of lignin to an easily grown and manipulated microbe, such as E. coli, allows for the breakdown of lignin on a large scale for the purpose of biofuel production. Lignin degradation would result in greater cellulosic fermentation efficiency by allowing easier access to cellulose and hemi-cellulose. The by products of lignin degradation could also be converted into a fuel, tapping yet another untapped abundant energy source. The white rot fungus, Phanerochaete chrysosporium, has the enzyme lignin peroxidase which seems to be promising for our purpose. If successful, a wide range of more efficient biofuel production methods will become available. This would not only result in increased fermentation efficiency of ethanol, but of various other fuels as well. Corn would also no longer have to be used as the plant source, eliminating much of the controversy and inefficiency from the process. Switch grass, wood scraps, yard waste, or just about any other plant matter could potentially be turned into fuel.
However, there are some difficulties with the implementation. Lignin is an extremely complex molecule formed by radical reactions, and it has proved very difficult to study. The mechanism of lignin peroxidase is not fully understood. Also, there could be other genes involved with lignin breakdown, some of which may not even be known to the experts.
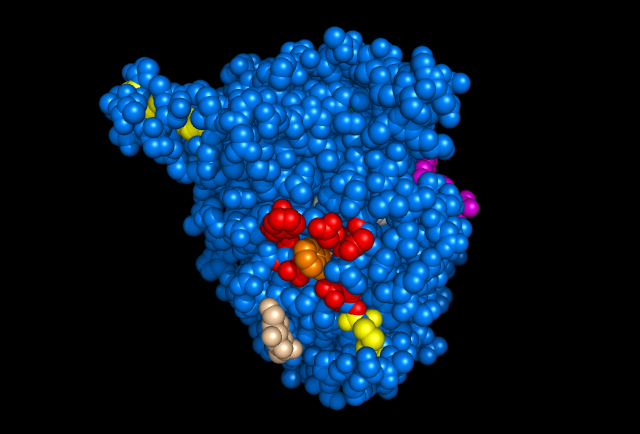
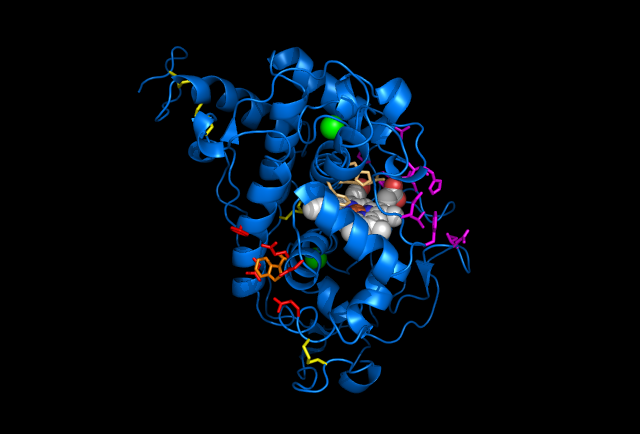
Figure 5: Images of Lignin peroxidase. Disulfide bonds (yellow), Ca2+ ions (green spheres), Heme access
channel (purple), Surface active cite (red) with hydroxylated typ171
(orange), Hydrogen peroxide active cite (tan wireframe, above heme),
Heme group (CPK coloring space fill.)
[http://www.youtube.com/watch?v=dYdru4ig5Q4 3D Video of Lignin peroxidase.]
In researching for an appropriate assay in analyzing the amount of lignin peroxidase produced by our E. coli strain, we found that the veratryl alcohol assay, developed in 1984, is currently the most widely used. It works by viewing the absorbance spectrum of the reaction solution. As veratryl alcohol is oxidized by lignin peroxidase over time, the optical density (OD) and wavelength of the absorbance peak change. By observing the disappearance of the substrate (the rate of change), we can determine the amount of active enzyme in the solution.
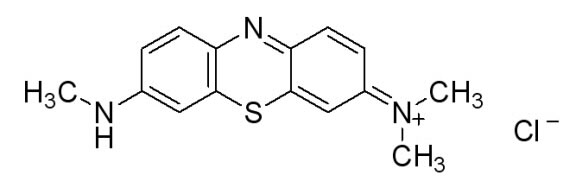
In doing additional research we came across a newer and seemingly more effective assay—the Azure B assay. Research showed that Azure B (a blue textile dye) has a higher specificity to lignin peroxidase compared to veratryl alcohol, as veratryl alcohol can be oxidized by fungal enyzmes other than lignin peroxidase. The Azure B assay works the same way as the veratryl alcohol assay in that the disappearance of the dye is measured to determine the amount of lignin peroxidase. The reaction solution comprises of a tartrate buffer (50 mM at pH 4.5), the dye Azure B (32 μM), hydrogen peroxide (100 μM), and the enzyme to be measured, lignin peroxidase.
Figure 6: [http://www.sigmaaldrich.com/catalog/search/ProductDetail/SIGMA/A4043 Azure B. Sigma Aldrich.] (2008). Retrieved Oct 18.
Lignin peroxidase has a few disulfide bonds, which are unable to form within E. coli. To combat this problem we used a strain (any of the Origami strains) available from Novagen that is a knockout for both thioredoxin reductase and glutathione reductase. This prevents the cell from reducing disulfide bonds as they form, allowing for eukaryotic proteins containing disulfide bonds to be expressed in the active form.
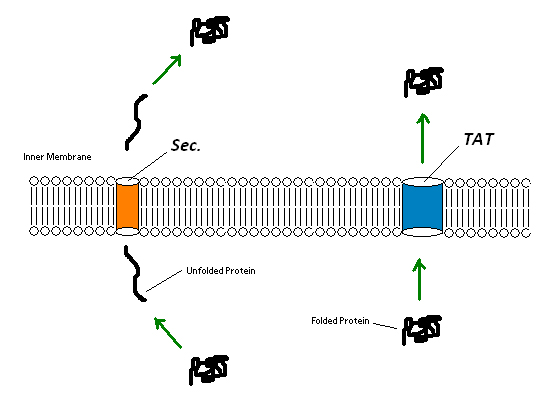
Since the extremely large plant cell wall complex cannot be imported into the cell we had to figure out a way to get lignin peroxidase out of the cell. This could either be done by lysing the cell and recovering the enzyme or by exporting the enzyme from the cell using a native export system. We looked into using two native export pathways in E. coli, the Sec pathway and the Twin Arginine Translocation (Tat) pathway. Proteins are recognized for transportation across the membrane by signal peptide sequences. The Sec pathway exports unfolded proteins, which then fold once in the periplasm. The Tat pathway exports already folded proteins. Since the cytoplasm within the Origami strains was an appropriate place for the protein to fold correctly we focused on the Tat pathway. Export of lignin peroxidase could be achieved by fusing sequences encoding for Tat signal peptides in frame to the lignin peroxidase gene.
Figure 7:TAT pathways can export entire folded proteins, while Sec involves an unfolding step, export of the unfolded protein and a refolding of the unfolded protein. Both pathways result in a folded protein in the periplasm.
References
Guzman L., Belin D., CarsonM., and Beckwith L. (1995). Tight regulation, modulation and high-level expression by vectors containing the arabinose pBAD promoter. Journal of Bacteriology. 177(14): 4121-4130.
Lorenzo Nissen, Gaspar Pérez-Martínez, María J. Yebra (2005) Sorbitol synthesis by an engineered Lactobacillus casei strain expressing a sorbitol-6-phosphate dehydrogenase gene within the lactose operon FEMS Microbiology Letters 249 (1) , 177–183 doi:10.1016/j.femsle.2005.06.010
Roe, A. J., C. O'Byrne, D. McLaggan, and I. R. Booth. 2002. Inhibition of Escherichia coli growth by acetic acid: a problem with methionine biosynthesis and homocysteine toxicity. Microbiology 148:2215-2222.
Ladero, V., Ramos, A., Wiersma, A., Goffin, P., Schanck, A., Kleerebezem, M., et al. (2007) High-level production of the low-calorie sugar sorbitol by Lactobacillus plantarum through metabolic engineering. Appl Environ Microbiol 73: 1864–1872.
Metzger J (2006) Production of liquid hydrocarbons from biomass. Angew. Chem. Int. Ed. 2006, 45, 696-698.
Lovingshimera, M. R., Siegeleb, D., & Reinharta, G. D. (2006). Construction of an inducible, pfkA and pfkB deficient strain of Escherichia coli for the expression and purification of phosphofructokinase from bacterial sources. Protein Expression and Purification , 46 (2), 475-482.
Karacaoğlan, V., & Özer, I. (2005). Steady-state kinetic properties of sorbitol dehydrogenase from chicken liver. Comparative Biochemistry and Physiology , 140, 309-312.
Archibald, F. S. A new assay for lignin-type peroxidases employing the dye azure B. Applied and Environmental Microbiology, 58(9), 3110-3116. "
"