Team:University of Lethbridge/Notebook/Project2October
From 2008.igem.org
m (→=Roxanne) |
m (→October 14, 2008) |
||
| Line 55: | Line 55: | ||
-Proceed with ligation. | -Proceed with ligation. | ||
| + | |||
| + | ===October 13, 2008=== | ||
| + | ====Roxanne==== | ||
| + | Ran a gel of the Freeze 'n Squeeze gel extraction. Some faint bands, but not enough confidence in the concentration to proceed with ligation. | ||
===October 14, 2008=== | ===October 14, 2008=== | ||
Revision as of 01:47, 30 October 2008
Back to The University of Lethbridge Main Notebook
Contents |
October 9, 2008
Roxanne, Munima, Christa, Sebastian
-Restriction digested RS3 and RS6 (produced by Nathan Puhl) and pSB1A2 + GFP sub with XbaI and SpeI
October 10, 2008
Roxanne
-Dephosphorylated pSB1A2 with Antarctic Phosphatase.
October 11, 2008
Roxanne
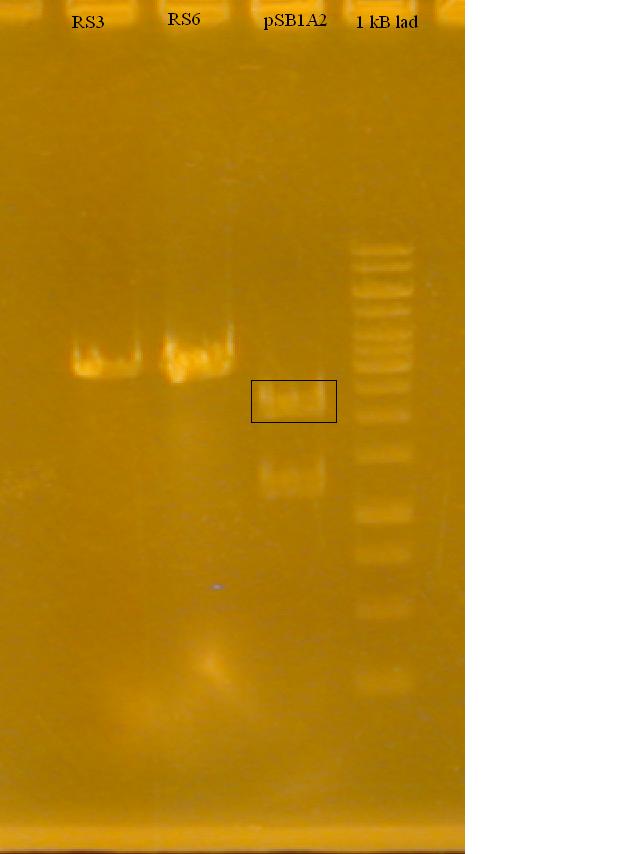
-Ran a gel to determine if the Restriction Enzymes cut the plasmid and RS3, RS6
October 12, 2008
Roxanne, Alix
-Restriction Digesting RS3 and RS6 again with XbaI and SpeI at 37.0 C for 2 hours.
-Attempting a new method of Gel Extraction (or rather an old method, new to us). Freeze 'n Squeeze.
Protocol: Freeze 'n Squeeze -Run the sample you wish to extract on a TAE-Agarose Gel -Cut out the band you wish to purify -Incubate in 1 gel volume 0.3 M NaCOOH at room temperature for 30 minutes. -Make your own spin column from a small microfuge tube with a hole cut out of the bottom, stuffed with glass wool. This tube should be inserted inside a 1.5 mL microfuge tube. -Transfer the solution to the spin column. -Freeze the tube in liquid Nitrogen for 1 minute, then spin at full speed for 15 minutes. -Precipitate the DNA in Ethanol. Remove the supernatant. -Wash in 75% Ethanol. Remove as much ethanol as possible. -Centrifige for 5 minutes then, remove the rest of the ethanol. -Let pellet to air dry for 10 minutes, this allows all ethanol to evaporate off. -Resuspend in TE Buffer, 10 uL. -Quantify either by Gel or UV Spec. -Proceed with ligation.
October 13, 2008
Roxanne
Ran a gel of the Freeze 'n Squeeze gel extraction. Some faint bands, but not enough confidence in the concentration to proceed with ligation.
October 14, 2008
Roxanne
Objective: PCR of the riboswitch in a 50 uL x 9 reactions.
Master Mix (1x):
-10x Buffer: 5 uL -10 mM dNTP: 1 uL - 50 mM MgCl2: 1.5 uL - 10 mM reverse primer: 1 uL -10 mM forward primer: 1 uL -Taq polymerase: 0.2 uL -d2H2O: 39.3 uL
October 15, 2008
Roxanne
Another PCR of the riboswitch in a 20 uL x 9 reactions.
October 16, 2008
Roxanne
-Ran gel of the PCR products. Some faint bands at the 2000bp level. The riboswitch is not amplifying.
October 17, 2008
Roxanne
-A synthetic riboswitch was ordered.
October 28, 2008
Roxanne
-Synthetic Riboswitch has arrived. Not enough time left to ligate into pSB1A2, transform and incubate on plates, subculture and plasmid prep in time to send away to the registry. 12 hours too short!!! It'll have to wait until next year. :(
 "
"