Team:Hawaii/Ligation of pRL1383a Parts
From 2008.igem.org
(Difference between revisions)
(8-14 ligation experimetn) |
(8-13 experiment, pcr conditions) |
||
| Line 31: | Line 31: | ||
<strong> Transformation </strong> | <strong> Transformation </strong> | ||
| + | |||
| + | :* | ||
<strong> Verification with Colony PCR </strong> | <strong> Verification with Colony PCR </strong> | ||
| + | :*Reaction conditions: 10ul volume: 5ul green taq, 4ul water, 1ul primers (VF2+VR)+ a sterile poke from a colony. | ||
| + | :*Running conditions: 94 hold, [95 30", 62 30", 72 (time varies based on size of verification site)]x 30 cycles, 72 10', 4 hold. | ||
| + | |||
== Results == | == Results == | ||
| Line 154: | Line 159: | ||
===8/13=== | ===8/13=== | ||
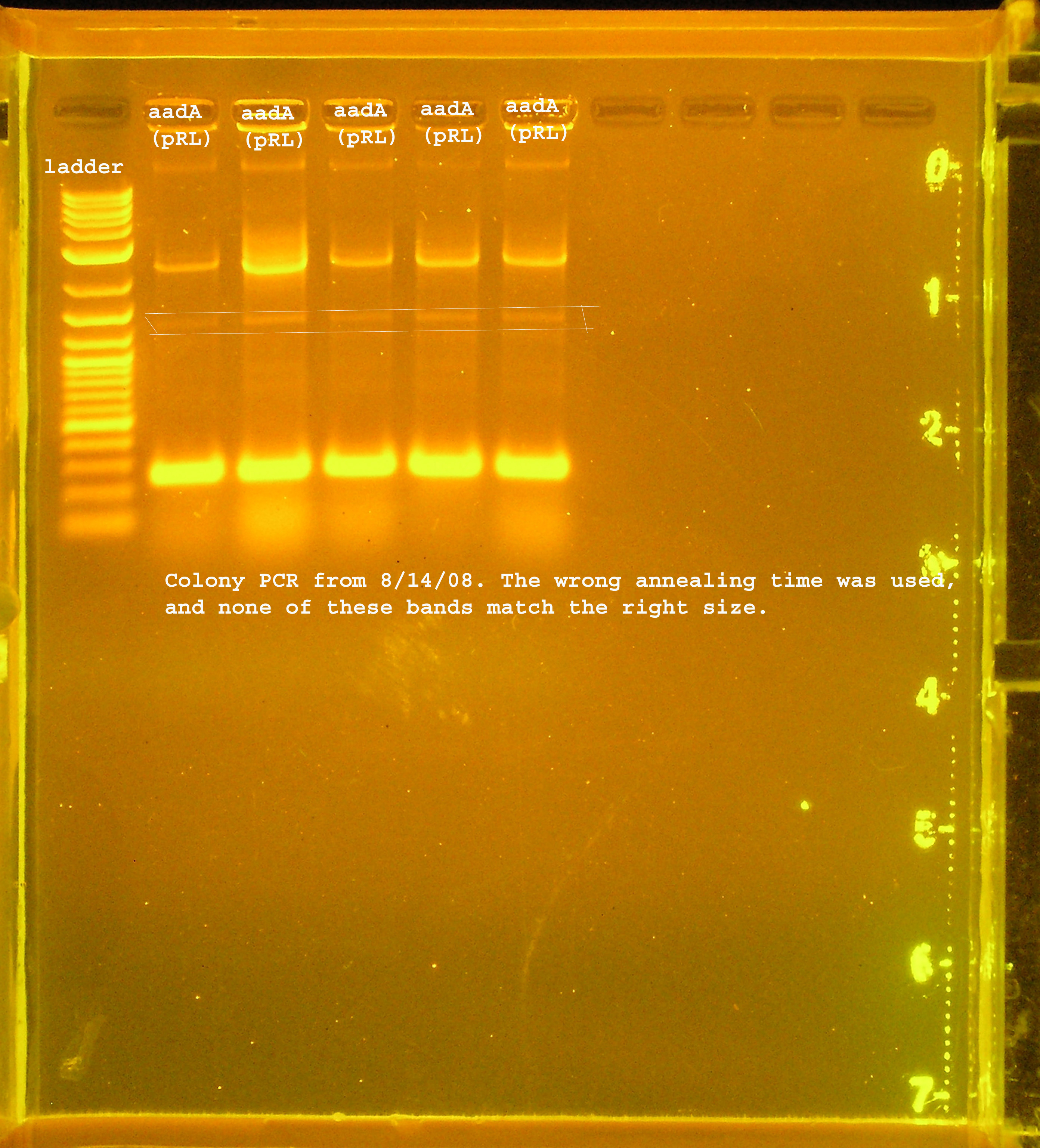
| - | [[Image:PCR_verification_8_14_08.jpg|right|thumb| | + | [[Image:PCR_verification_8_14_08.jpg|right|thumb|150px|PCR verification of rep, P1lytic, and aadA from pRL1383a from 8/14/08.]] |
| + | [[Image:PCR_verification_aada_8_14_08.jpg|right|thumb|150px|PCR verification of aadA from pRL1383a.]] | ||
:*Three ligations were performed today. I checked my math from the last ligation and decided it wasn't totally correct for the rep ligation to B0030. | :*Three ligations were performed today. I checked my math from the last ligation and decided it wasn't totally correct for the rep ligation to B0030. | ||
:*P1 lytic was ligated to B0015 (TT), Rep to B0030 with precisely 2:1 insert to vector ratio, and the aadA region from pRL1383a to B0030. | :*P1 lytic was ligated to B0015 (TT), Rep to B0030 with precisely 2:1 insert to vector ratio, and the aadA region from pRL1383a to B0030. | ||
| Line 162: | Line 168: | ||
:*A lawn of colonies was observed for all three transformations. No colonies grew for either control (need to check pUC18 and see if there are problems with our stock). | :*A lawn of colonies was observed for all three transformations. No colonies grew for either control (need to check pUC18 and see if there are problems with our stock). | ||
<strong>PCR Verification</strong> | <strong>PCR Verification</strong> | ||
| - | :* | + | :*The rep region, as with the last ligation was not inserted to the vector. The band at 300bp suggest the B0030 vector was amplified by VF2&VR. Another possibility is that some of this plasmid was not digested and the ligation just did not work, so these were the only circular plasmids available for transformation. |
| + | :*The P1 lytic region is at 400kb while the verification size of B0015 is 445bp... | ||
| + | :*aadA from pRL1383a is amplified to several different sizes. One of which is about correct (~1kb), one above 10kb, and one at 0.25kb. | ||
Revision as of 23:53, 21 August 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Contents |
Ligation of Parts
- The BioBrick parts of pRL1383a are to be ligated in a series of experiments.
Methods
Restriction Digest
- Each part is digested with both enzymes. For all cases NEBuffer 2 is used.
- Reaction Conditions:
- 5ul Buffer
- 1ul Enzyme 1 + 1ul Enzyme 2
- 0.5 ul BSA
- Xul water
- Yul insert (1ug if possible)
- Zul vector (less than 1ug)
- Running Conditions: 2 hours at 37°C.
- Check the progress of the reaction by running a 0.8% gel.
Ligation
- Refer to http://openwetware.org/wiki/DNA_ligation for the reasoning behind this.
Transformation
Verification with Colony PCR
- Reaction conditions: 10ul volume: 5ul green taq, 4ul water, 1ul primers (VF2+VR)+ a sterile poke from a colony.
- Running conditions: 94 hold, [95 30", 62 30", 72 (time varies based on size of verification site)]x 30 cycles, 72 10', 4 hold.
Results
7/27
- A few re-digests and ligations were performed today: oriT + pSB1A3, oriV + pSB1A3, mob + B0024, rep + R0010, aadA(pRL1383a+R0010). Afterwards, the ligation product was transformed to DH5-alpha. Only OriT transformed. I did not check the progress of this experiment with a gel, so I am not sure where this went wrong.
- Next time, a gel will be run after every step.
| Name | size | enzyme | quantity |
|---|---|---|---|
| oriT | ~125bp | XbaI & PstI | n/a |
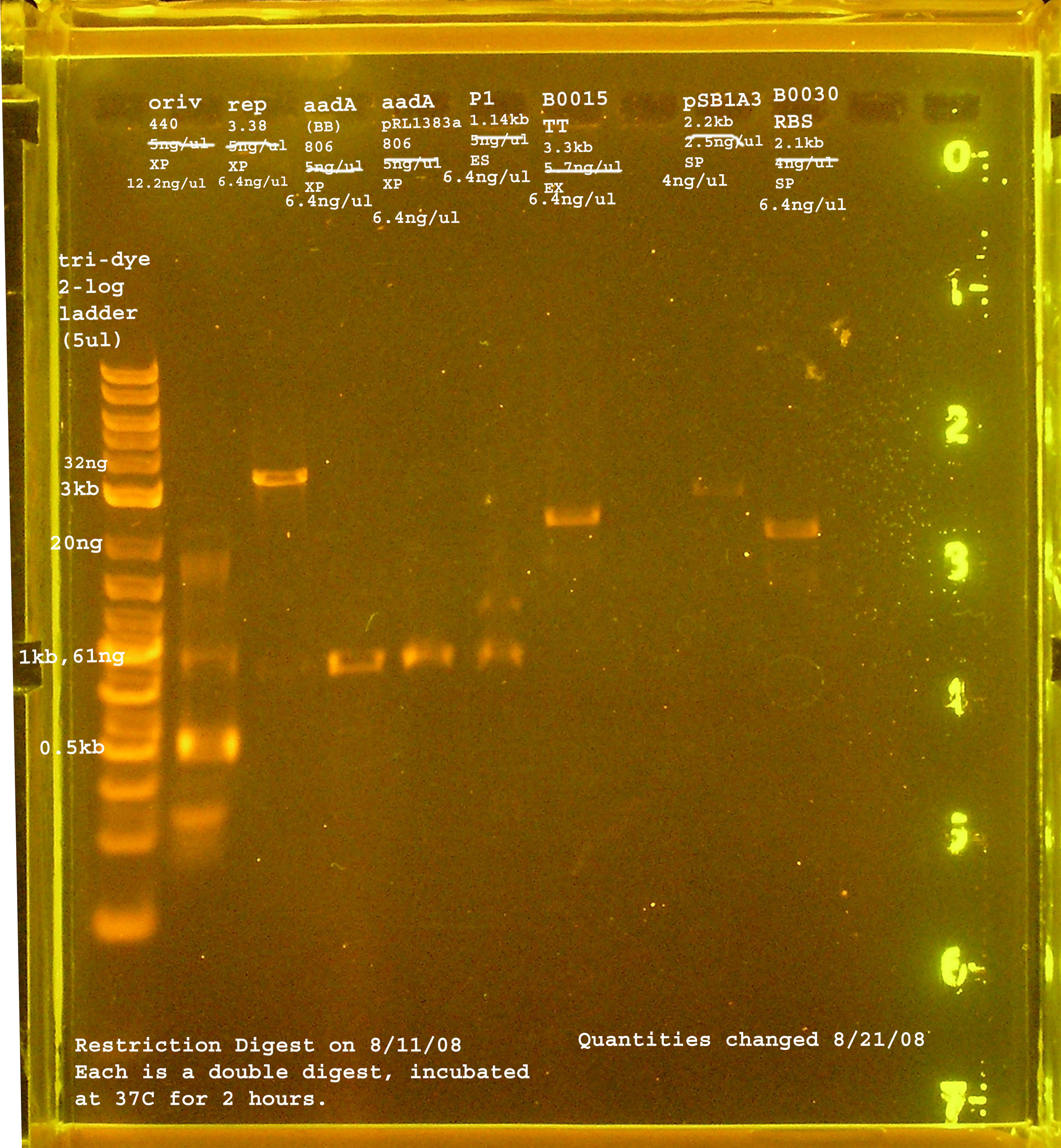
8/11
| Name | size | enzyme | quantity |
|---|---|---|---|
| rep | 3.3kb | XbaI & PstI | 5ng/ul |
| oriV | 415bp | XbaI & PstI | 5ng/ul |
| aadA (pRL1383a) | 806bp | XbaI & PstI | 5ng/ul |
| aadA (BB) | 806bp | XbaI & PstI | 5ng/ul |
| P1 lytic Region | 1.3kb | EcoRI & SpeI | 5ng/ul |
| [http://partsregistry.org/Part:BBa_B0030 BBa_B0030] | 2094bp | SpeI & PstI | 4ng/ul |
| [http://partsregistry.org/Part:BBa_B0015 BBa_B0015] | 3318bp | EcoRI & XbaI | 5.7ng/ul |
| [http://partsregistry.org/Part:pSB1A2 pSB1A2] | 2094bp | SpeI & PstI | 5.5ng/ul |
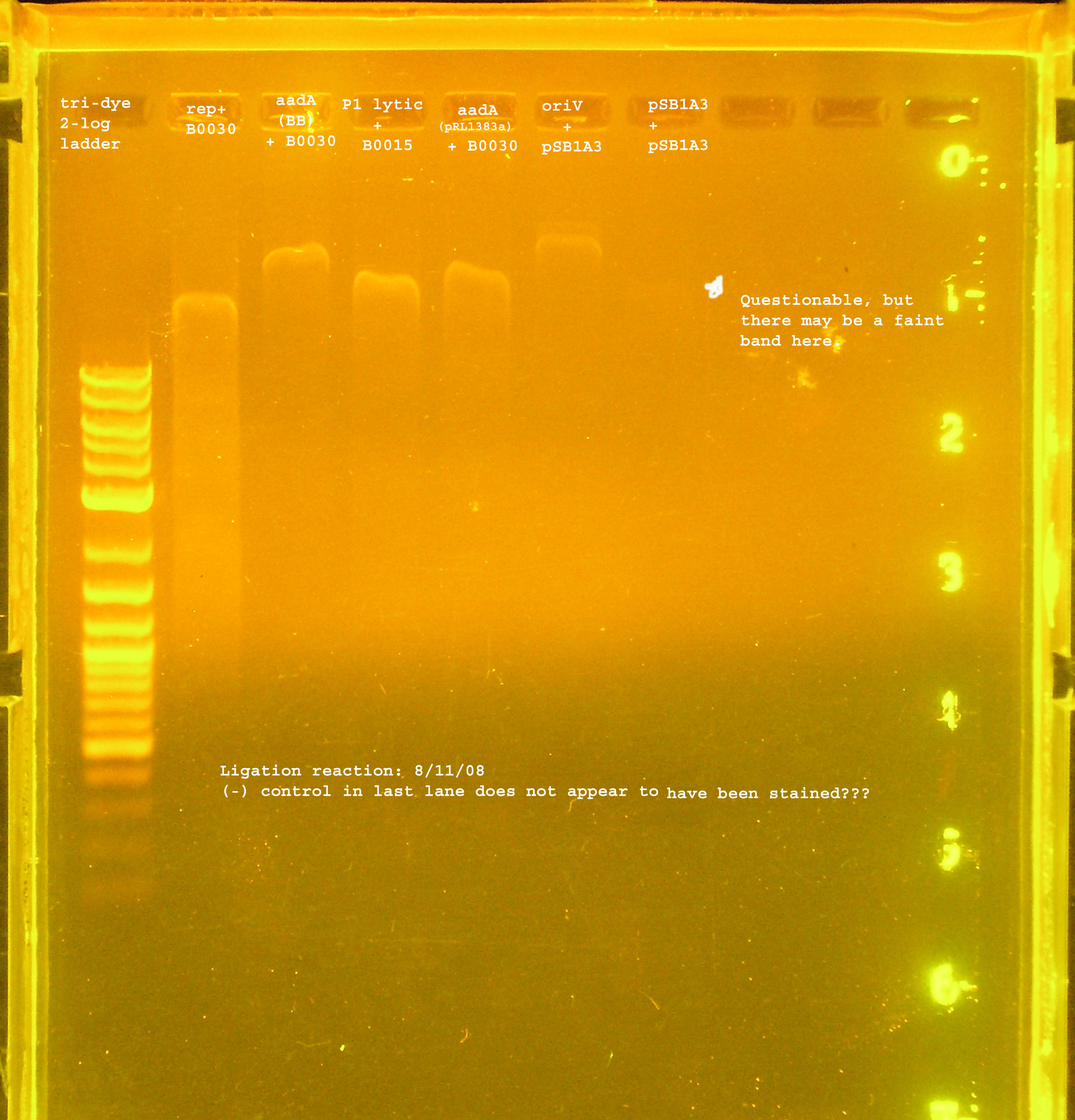
| Name | strain, antibiotics | colonies? after next day??? | PCR verification in bp theoretical, experimental |
|---|---|---|---|
| rep(20ng)+B0030(16ng) | DH5-alpha & Amp100 | lawn | 3.3kb, 300&600 |
| oriV(20ng)+pSB1A2(50ng) | DH5-alpha & Amp100 | 21 colonies | 676, 600 |
| aadA (BB)+ B0030 | DH5-alpha & Amp100 | lawn | 900, #4:900, others: 300 |
| aadA(pRL1383a)+B0030 | DH5-alpha & Amp100 | lawn | 900, 300 |
| P1 lytic + B0015 | DH5-alpha & Kan50 | no colonies | n/a |
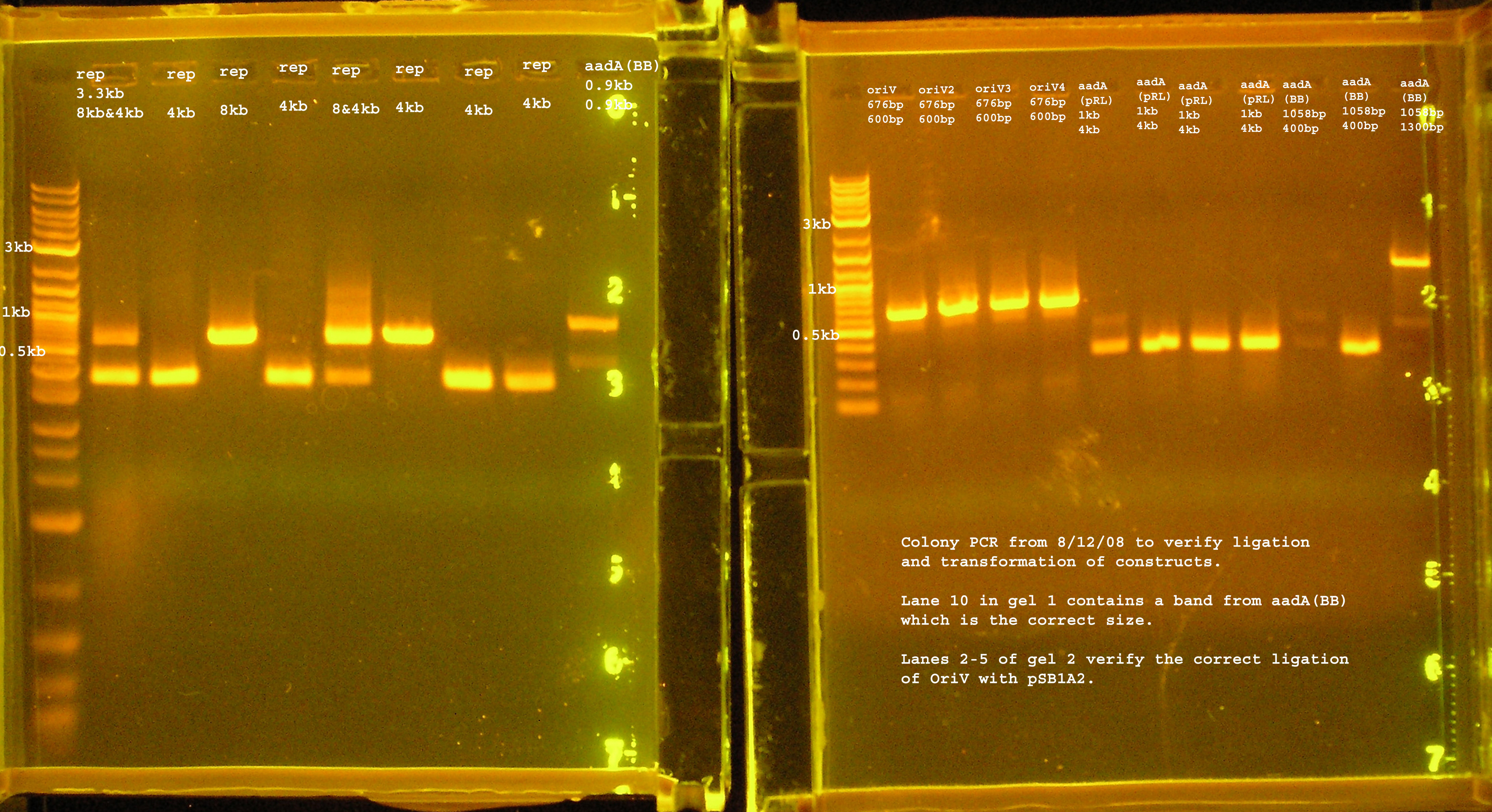
Transformation & Verification PCR
- The only bands corresponding to the correct size are the 4 oriVs and the 4th BB version of aadA.
- The rep region lanes are all either 300 or 600bp when they should be 3.3kb. If the RBS (B0030) ligated back to itself, the band should be 253bp. The bands at 300bp may be explained by this. It is unclear what happened in the latter case. The incorrect aadA bands are also around 300bp which would mean the RBS containing vector ligated back to itself some how. B0030 was cut with SpeI and PstI.
Plasmid Prep
- OriV1-4 and aadA (BB) were cultured over-night in Terrific Broth with amp100 as selection.
- The plasmids were isolated using an Alkaline Lysis Mini-Prep.
8/13
- Three ligations were performed today. I checked my math from the last ligation and decided it wasn't totally correct for the rep ligation to B0030.
- P1 lytic was ligated to B0015 (TT), Rep to B0030 with precisely 2:1 insert to vector ratio, and the aadA region from pRL1383a to B0030.
- Unfortunately there is no gel to confirm this ligation.
Transformation
- The ligation products were transformed into DH5-alpha cells with selection on LB amp100 plates. pUC18 was transformed into DH5-alpha cells as a positive control, while no plasmid was used in a transformation as a negative control.
- A lawn of colonies was observed for all three transformations. No colonies grew for either control (need to check pUC18 and see if there are problems with our stock).
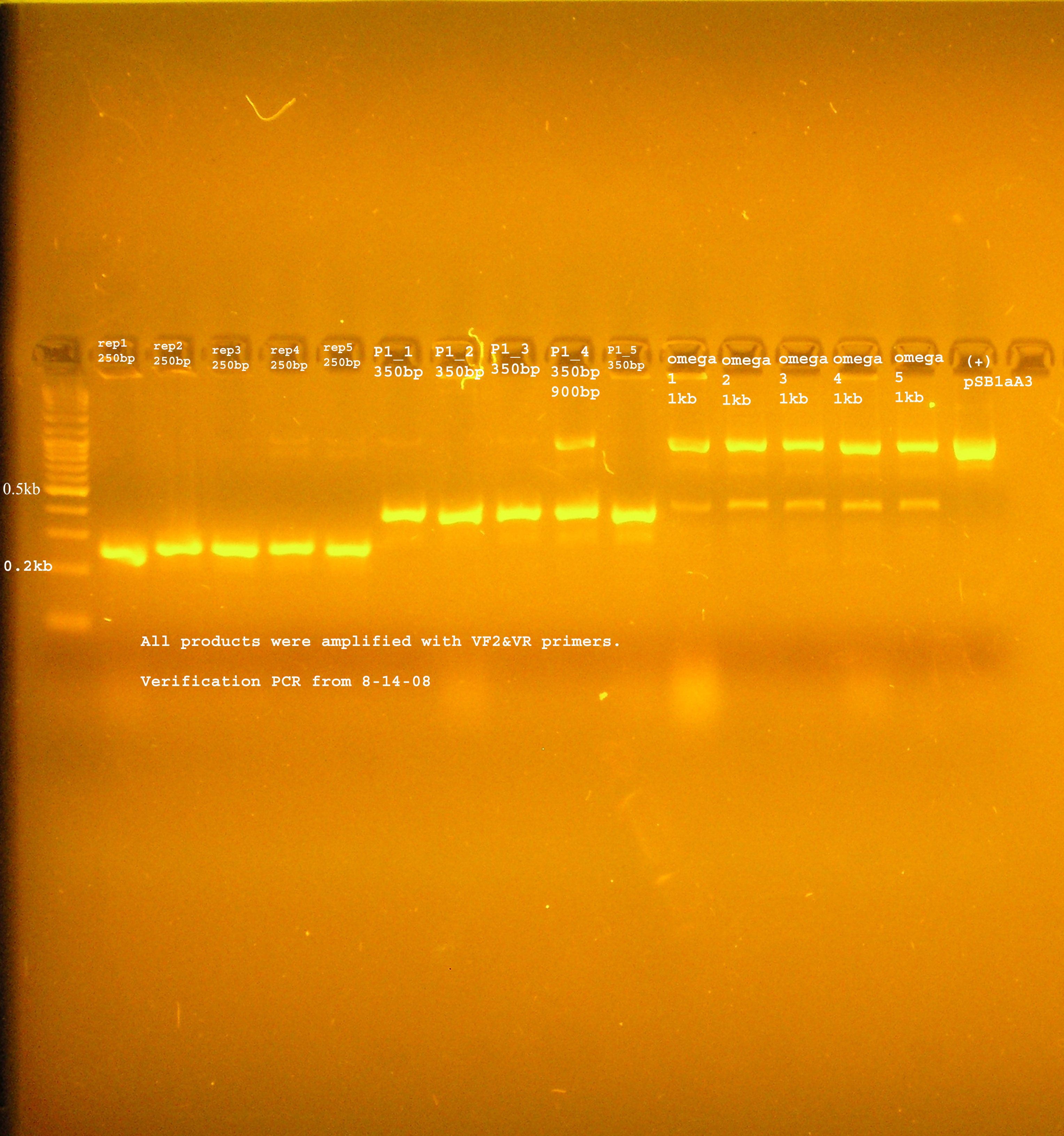
PCR Verification
- The rep region, as with the last ligation was not inserted to the vector. The band at 300bp suggest the B0030 vector was amplified by VF2&VR. Another possibility is that some of this plasmid was not digested and the ligation just did not work, so these were the only circular plasmids available for transformation.
- The P1 lytic region is at 400kb while the verification size of B0015 is 445bp...
- aadA from pRL1383a is amplified to several different sizes. One of which is about correct (~1kb), one above 10kb, and one at 0.25kb.
Discussion
- What was learned and how to do future experiments differently.
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"