Complexations Caracterisation
The first hypothesis is that a complexation reaction is fully determined by the following :
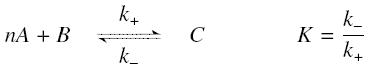
and that the rates k+ et k- stay constant under all conditions. Then, the second hypothesis is that these equations are (kinetically speaking) elementary :
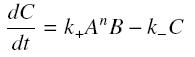 and at steady-state :
and at steady-state : 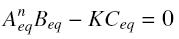
Then, since we guess that the only datas we will have are the quantities of A and B introduced (Ai and Bi), the only equations we will deal with is the following, entirely determining the concentration Ceq at steady-state (at least if we take the smallest real root of the equation, it is useless to demonstrate the unicity, or even the existence, of such a solution) :
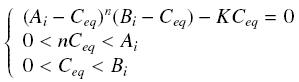
Equilibrium of a Complex
Know, if we imagine a given amount of Ai and Bi, that are calculated as their equilibrium without taking acount of their complexation (but, for instance, of other interactions, productions and disappearance), and that the produced complex C disappears along time with a degradation rate γ, we get :
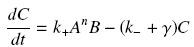
so that the equilibrium gives :
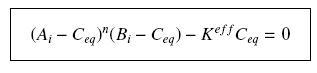
with
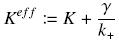
Hill Functions
The previous system of complexation applied in particular to the association of the Promoters (P) and its Transcription Factor (TF).
Because the promoters on a "low copy plasmid" exists in the cell in ten exemplaries, in contrary to a protein, which, as long as it is produced (even weakly) exists in thousands of exemplaries, we assume can the quantities of TF and P are different by several orders of magnitude. Then, with the previous notations, if A, B and C stands respectively for TF, P and the complex TF><P, we will get
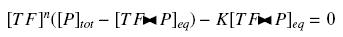
that we can easily solve with :
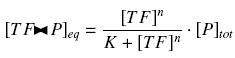
Depending to the order n (also called cooperativity, because it correponds to the possibilities of the transcription factor to binds in a group on the promoter), this function is a sigmoïd, known as the Hill function. The parameter K , called activation constant, is often replaced in the previous expression by the following notation
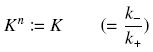
It simplifies the manipulations of the expression ; we can notice that K represents now the amount of TF needed to bind half of the total P in the cell.
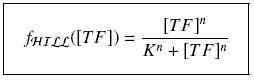
Induction by a small molecule
Introduction
In certain steps of our system, and in our caracterisation plan, we use the diffusion of a small molecule (SM), which, bound to a protein, will act as a transcription factor.
We make the hypothesis of a simple, passive diffusion, that leads at the steady-state at equal amount of the small molecule, inside and outside the cells. The resulting equations are the following coupling :
where
- permeability is the membrane permeability multiplied by the average external surface of a cell (in L)
- N is the average cell population
- Vint is the average volume of a cell (in L) ; Vext is the volume of the culture medium outer the cells (in L)
The situation
The involvement of the previous process is, in our systems,under these conditions :
- Prot is a protein, produce by a promoter
- expr_prom is the given expression of this promoter (production rate of Prot)
- γ is the degradation/dilution rate of the proteins and complexes in the cell (especially due to cell division, we made so far the hypothesis that it is the same for all the proteins)
- SMint binds to Prot, with a cooperativity n and a dissociation constant Kd, to form the complex Compl
- The cell culture is in a chemostat : the cell population is N; The renewal rate (flow of the medium, in L.min-1) is rnw; The Volume of a cell is Vint; and of the outer medium is Vext ( = Volume(chemostat) - N * Vint)
Induction for the caracterisations
see details on the induction
In this experimental conditions, the input medium has a constant (controlled) concentration [SM]i. So, we can easily make the hypothesis that it impose the [SM]ext in the outer medium ; and, under the hypothesis of a "quick enough" diffusion, we get at steady-state :
Then, considering the previous complexations equations, and by the equilibrium of Prot, we have at steady states
and by the definition of the dissociation constant
that leads to the following expression of the complexes and proteins :
Then, if one of the precious meloecules are transcription factor for a given promoter, by considering the previous Hill functions (with cooperativity m and dissociation constant KHILL and the amount of [SM]i, we have the following expressions :
Finding Parameters
Then, by solving the complexation equation for a given set of K et n (and, in term of production of protein, the b (or β) parameter (see hypothesis)), we can have an approximation of the corresponding Ceq. The idea, in order to find the bests parameters which correspond to our measurements, is to run a basical program of quadratic optimisation. The final error would tell us if our modelisation is consistent.
((((the following is not coresponding to the new model, but we will use the same frame to expose the corresponding program)))))
(((((((This program aims at fill this data bank, from experimental data obtained by our wet lab. )))))))
(((((( After some trials, we have obtained interesting results in terms of precision. We are now trying to test the robustness of the estimations in order to quantify the influence of biological variance in the results as well as the influence of the number of points available. ))))))))
((((( )))))
(((((( The corresponding code can be found there : Estimation of the parameters )))))))
|