Team:Heidelberg/Notebook/Killing I/Notebook/week12
From 2008.igem.org


| << Week 11 | Overview | Week 13 >> |
|---|
Week 12
Contents |
Monday, 10/20/08
Proceedings of phage cloning strategy one
- the fully mutated insert in pBluescript SK II was digested with XbaI/XhoI and the insert extracted from the gel
- lambda DNA was also cut with XbaI/XhoI and the backbone also extracted from the gel.
- the insert was ligated in the lambda backbone over night using standard ligation methods
Phage cloning strategy two
- Digestion with XbaI/XhoI
- should be: 3945, 2898
- Gel
- lane 1-8: new minipreps
- lane 9: old insert fully mutated
- results: the new minipreps seem to be the old unmutated pBluescript
Characterization of oriT
- Quantitatively test for oriT
Donor: overnight culture Top10 oriT+pUB307 OD(600nm): 1.548
Recipient:
overnight culture Top10 pBAD 33 OD(600nm): 1.808
overnight culture MG1655 pBAD 33 OD(600nm): 2.932
overnight culture DH5alpha pBAD 33 OD(600nm): 1.976
- Centrifuge overnight culture in 1.5ml eppi for 2min at 13000rpm, 24 samples donor, 510ul/sample; 8samples Top10 recipient, 450ul/sample; 8samples MG1655 recipient, 375ul/sample; 8samples DH5alpha recipient, 600ul/sample;
- Wash the pellet twice with LB medium
- Resolve the pellet in LB medium
- Centrifuge the washed recipient for 2min at 13000rpm, discard the fluid
- Add the washed donor suspension
- Vortex and resolve the pellet
- Centrifuge the mix for 1min at 13000rpm
- Resolve the pellet in 100ul LB
- Put membrane filter on the LB agar
- Pipett the suspension on membrane filter (8samples*3tests)
- Incubate the plates with membrane filter at 37°C
- Put directly one membrane filter into 1ml LB in an 1.5ml eppi
- Vortex the eppi for 30sec, dilute for 10-5 and 10-6, plate them out on LB/Amp+Cm plates (0min)
- For Top10 as recipient: plate them out also on LB/Kan+Cm plates and LB/Amp+Kan+Cm plates
- After 6, 12, 18, 24, 30, 36, 42 min repeat the last three steps.
- Negative control plates:
- LB/Cm+Amp:
- LB/Cm+Amp:
100ul donor overnight culture
100ul recipient overnight culture (x3)
- Cell number determination
- LB/Cm: 100ul 10-6 recipient overnight culture (x3)
- LB/Kan+Amp: 100ul 10-6 donor overnight culture
- Cell number determination
- Result:
- Negative control: negative
- Colony on LB/Cm:
Top10:166 (Titer of recipient: 1.66e9/ml)
MG1655:194 (Titer of recipient: 1.94e9/ml)
DH5alpha:115 (Titer of recipient: 1.15e9/ml)
- Colony on LB/Kan+Amp: 140 (Titer of donor: 1.4e9/ml)
- Colony on LB/Kan+Amp: 140 (Titer of donor: 1.4e9/ml)
(information for volume counting: 1OD Top10 = 9e8/ml; 1OD MG1655 = 6.6e8/ml; 1OD DH5alpha = 5.8e8/ml)
- Colony on other LB/Cm+Amp plates, LB/Kan+Cm plates and LB/Amp+Kan+Cm plates:
- 10-5 dilute:
- 10-5 dilute:
- Colony on other LB/Cm+Amp plates, LB/Kan+Cm plates and LB/Amp+Kan+Cm plates:
| Time | Top10-CA | Top10-CK | Top10-CKA | MG1655 | DH5alpha |
|---|---|---|---|---|---|
| 0 | 37 | 2 | 1 | 0 | 7 |
| 6 | 390 | 17 | 4 | 1 | 22 |
| 12 | 1390* | 194 | 90 | 7 | 480 |
| 18 | 1720* | 230 | 109 | 49 | 1180* |
| 24 | 2880* | 1130* | 800* | 136 | 1920* |
| 30 | 3500* | 2500* | 1540* | 260 | 2960* |
| 36 | 3750* | 2600* | 1800* | 920* | 3440* |
| 42 | 960* | 4040* |
'*':10-6 dilute
Tuesday, 10/21/08
Proceedings of phage cloning strategy one
- the emerging plasmid consisting of our insert in tha lambda phage was transformed in E. coli by chemical transformation and in vitro packaging
- the bacterias were plated on chloramphenicol and incubated at 28 °C to ensure that the thermolabile cI (cI857) was not degraded
Phage cloning strategy two
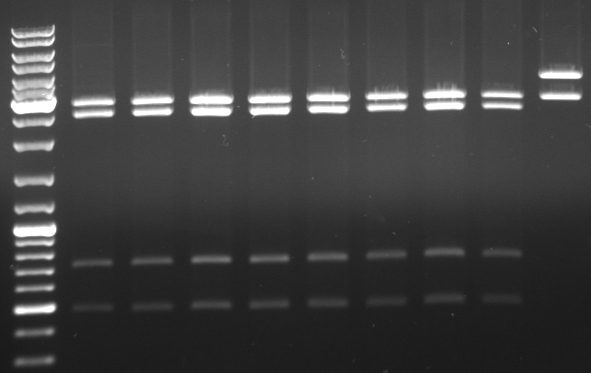
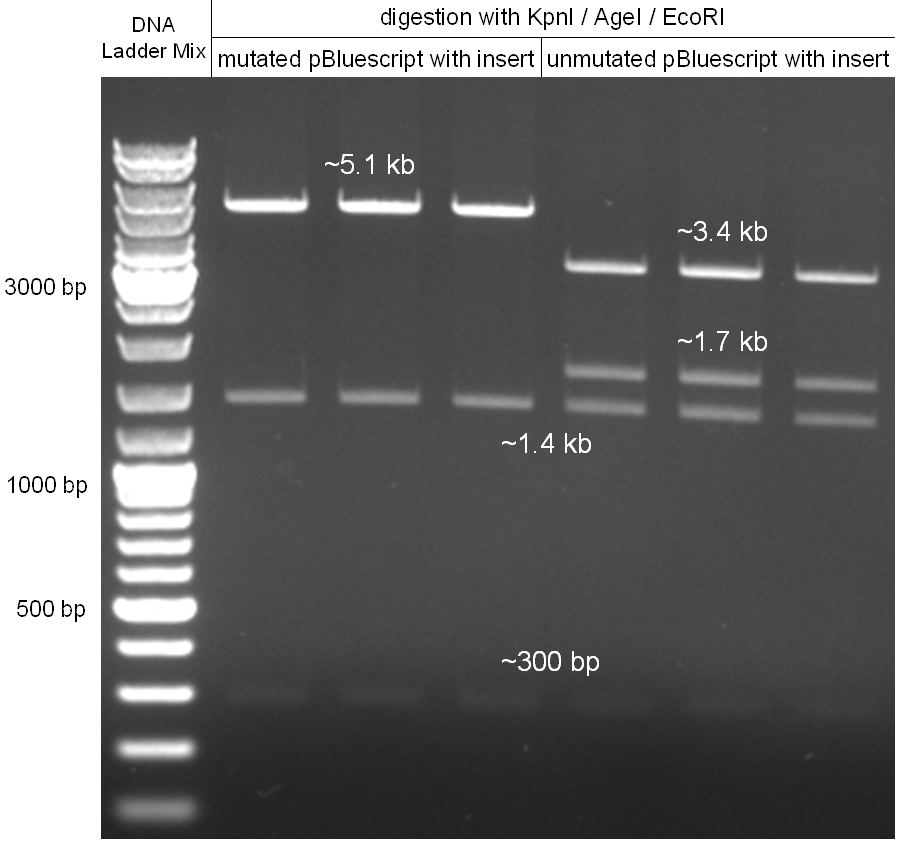
- do again a digestion of mutagenesis pcr minipreps from 10/07/08 but with an additional restriction enzyme (EcoRI)to get a better separation on gel to cut out the backbone
- digestion with KpnI/AgeI/EcoRI
- mutagenesis pcr succesful: 300bp, 1.4kb 5.1kb
- mutagenesis pcr not succesful: 300bp, 1.4kb, 1.7kb, 3.4kb
- -->mutagenesis pcr on the first three lanes successful
- cut out 5.1kb band and gel purification kit
- lane0: DNA ladder mix
- lane1: purified 5.1kb band from last gel (pBluescript backbone from phage cloning strategy one (KpnI/AgeI)
- lane2: CmR1 digested (KpnI/SacI)
- lane3: CmR2 digested (KpnI/SacI)
- lane4: GFP1 digested (SacI/AgeI)
- lane5: GFP2 digested (SacI/AgeI)
- ligation of purified 5.1kb band from last gel (pBluescript backbone from phage cloning strategy one (KpnI/AgeI) with CmR1/2 and GFP1/2
- 30min at room temperature
- transformation in TOP10
Characterization of oriT
- Inoculate the cells for conjugation test
15ml LB/chloramphenicol + glycerol stock Top10 pBAD 33 (x2)
15ml LB/kanamycin/ampicilin +glycerol stock Top10 oriT+pUB307 (x2)
Wednesday, 10/22/08
Proceedings of phage cloning strategy one
- first small colonies can be seen on chloramphenicol plates.
- colonies were picked and transferred in liquid media containing chloramphenicol
Characterization of oriT
- Qquantitatively test for oriT (twice)
Donor 1: overnight culture Top10 oriT+pUB307 OD(600nm): 2.828
Donor 2: overnight culture Top10 oriT+pUB307 OD(600nm): 3.004
Recipient 1: overnight culture Top10 pBAD 33 OD(600nm): 3.116
Recipient 2: overnight culture Top10 pBAD 33 OD(600nm): 3.084
- Centrifuge overnight culture in 1.5ml eppi for 2min at 13000rpm, 15samples donor1, 15samples recipient1, in different volume (see the result table below)
- Wash the pellet twice with LB medium
- Resolve the pellet in LB medium
- Centrifuge the washed recipient for 2min at 13000rpm, discard the fluid
- Add the washed donor suspension
- Vortex and resolve the pellet
- Centrifuge the mix for 1min at 13000rpm
- Resolve the pellet in 36ul LB
- Put membrane filter on the LB agar
- Pipett the suspension on membrane filter (15samples)
- Incubate the plates with membrane filter at 37°C for 20min
- Put membrane filter into 1ml LB in an 1.5ml eppi
- Vortex the eppi for 30sec, dilute for 10-6, plate them out on LB/Amp+Cm plates
- Negative control plates:
- LB/Cm+Amp:
- LB/Cm+Amp:
100ul donor 1 overnight culture
100ul recipient 1 overnight culture
- Cell number determination
- LB/Cm: 100ul 10-6 recipient 1 overnight culture
- LB/Kan+Amp: 100ul 10-6 donor 1 overnight culture
- Cell number determination
- Repeat the test with donor 2 and recipient 2.
- Result:
- Negative control: negative
- Colony on LB/Cm:
recipient 1: 458 (Titer of recipient: 4.58e9/ml)
recipient 2: 403 (Titer of recipient: 4.03e9/ml)
Colony on LB/Kan+Amp:
donor 1: 347 (Titer of donor: 3.47e9/ml)
donor 2: 288 (Titer of donor: 2.88e9/ml)
- Reaction volume and colony on other LB/Cm+Amp plates:
- Reaction volume and colony on other LB/Cm+Amp plates:
| Sample | Donor 1(ul) | Rezipient 1(ul) | colony 1 | Donor 2(ul) | Rezipient 2 (ul) | colony 2 |
|---|---|---|---|---|---|---|
| 50:1 | 289 | 5.1 | 12 | 289 | 5,2 | 16 |
| 25:1 | 283 | 10 | 28 | 283 | 10.1 | 31 |
| 10:1 | 268 | 24 | 62 | 253 | 24 | 63 |
| 8:1 | 262 | 29 | 81 | 247 | 29 | 75 |
| 5:1 | 246 | 44 | 115 | 231 | 44 | 120 |
| 3:1 | 221 | 66 | 161 | 208 | 66 | 129 |
| 2:1 | 196 | 88 | 213 | 185 | 88 | 212 |
| 1:1 | 147 | 132 | 302 | 139 | 132 | 303 |
| 1:2 | 98 | 175 | 351 | 93 | 175 | 298 |
| 1:3 | 74 | 197 | 294 | 69 | 197 | 302 |
| 1:5 | 49 | 219 | 289 | 46 | 219 | 194 |
| 1:8 | 35 | 234 | 241 | 31 | 234 | 190 |
| 1:10 | 27 | 239 | 158 | 25 | 239 | 134 |
| 1:25 | 11.3 | 253 | 86 | 10.7 | 253 | 82 |
| 1:50 | 5.8 | 258 | 31 | 5.4 | 258 | 30 |
- Inoculate the cells for conjugation test
15ml LB/chloramphenicol + glycerol stock Top10 pBAD 33 (x2)
15ml LB/kanamycin/ampicilin +glycerol stock Top10 oriT+pUB307 (x2)
Thursday, 10/23/08
Proceedings of phage cloning strategy one
- colonies could grow in chloramphenicol containing medium --> samll aliquots were transferred in ampicillin containing medium to check for the resisitance
- colony PCRs were conducted to ensure that our insert is embedded in the growing cells
- pUB307 was transferred in cells harboring our modified lambda phage by conjugation --> plating on Cm + Kan
phage cloning strategy two
- inoculation of liquid cultures from ligation (tuesday)
Characterization of oriT
- Quantitatively test for oriT (twice)
Donor 1: overnight culture Top10 oriT+pUB307 OD(600nm): 1.404
Donor 2: overnight culture Top10 oriT+pUB307 OD(600nm): 1.288
Recipient 1: overnight culture Top10 pBAD 33 OD(600nm): 1.428
Recipient 2: overnight culture Top10 pBAD 33 OD(600nm): 1.292
- Centrifuge overnight culture in 1.5ml eppi for 2min at 13000rpm, 15samples donor1, 280ul/sample; 15samples recipient1, 530ul/sample;
- Wash the pellet twice with LB medium
- Resolve the pellet in LB medium
- Centrifuge the washed recipient for 2min at 13000rpm, discard the fluid
- Add the washed donor suspension
- Vortex and resolve the pellet
- Centrifuge the mix for 1min at 13000rpm
- Resolve the pellet in 40ul LB
- Put membrane filter on the LB agar
- Pipett the suspension on membrane filter (15samples)
- Incubate plates with membrane filter at different temperature for different time
| 4°C | 22°C | 30°C | 37°C | 42°C | |
|---|---|---|---|---|---|
| 20min | 1plate | 1plate | 1plate | 1plate | 1plate |
| 40min | 1plate | 1plate | 1plate | 1plate | 1plate |
| 60min | 1plate | 1plate | 1plate | 1plate | 1plate |
- Put membrane filter after the incubation into 1ml LB in an 1.5ml eppi
- Vortex the eppi for 30sec, dilute for 10-6, plate them out on LB/Amp+Cm plates
- Negative control plates:
- LB/Cm+Amp:
- LB/Cm+Amp:
100ul donor 1 overnight culture
100ul recipient 1 overnight culture
- Cell number determination
- LB/Cm: 100ul 10-6 recipient 1 overnight culture
- LB/Kan+Amp: 100ul 10-6 donor 1 overnight culture
- Cell number determination
- Repeat the test with donor 2 (300ul/sample) and recipient 2 (590ul/sample).
- Result:
- Negative control: negative
- Colony on LB/Cm:
recipient 1: 138 (Titer of recipient: 1.38e9/ml)
recipient 2: 99 (Titer of recipient: 0.99e9/ml)
- Colony on LB/Kan+Amp:
- Colony on LB/Kan+Amp:
donor 1: 129 (Titer of donor: 1.29e9/ml)
donor 2: 110 (Titer of donor: 1.1e9/ml)
- Colony on other LB/Cm+Amp plates:
- Donor 1 + recipient 1
- Donor 1 + recipient 1
- Colony on other LB/Cm+Amp plates:
| 4°C | 22°C | 30°C | 37°C | 42°C | |
|---|---|---|---|---|---|
| 20min | 5 | 2 | 71 | 154 | 167 |
| 40min | 5 | 78 | 178 | 285 | 273 |
| 60min | 1 | 117 | 241 | 550 | 402 |
- Donor 2 + recipient 2
- Donor 2 + recipient 2
| 4°C | 22°C | 30°C | 37°C | 42°C | |
|---|---|---|---|---|---|
| 20min | 2 | 29 | 43 | 118 | 122 |
| 40min | 1 | 61 | 117 | 290 | 271 |
| 60min | 0 | 79 | 227 | 332 | 234 |
Friday, 10/24/08
Proceedings of phage cloning strategy one
- colonies grew on plates containing chloramphenicol and kanamycin --> were picked and liquid medium inoculated
- conjugation of our lambda phage into cells harboring T9002 in pSB1A3 --> brought out on plates containing chloramphenicol and ampicillin
phage cloning strategy two
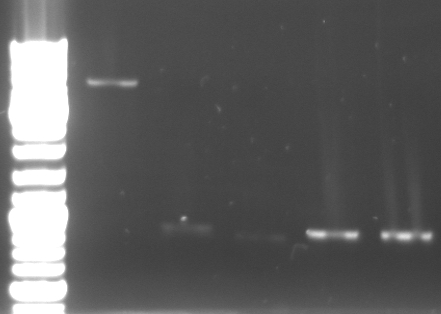
- Miniprep of overnight cultures
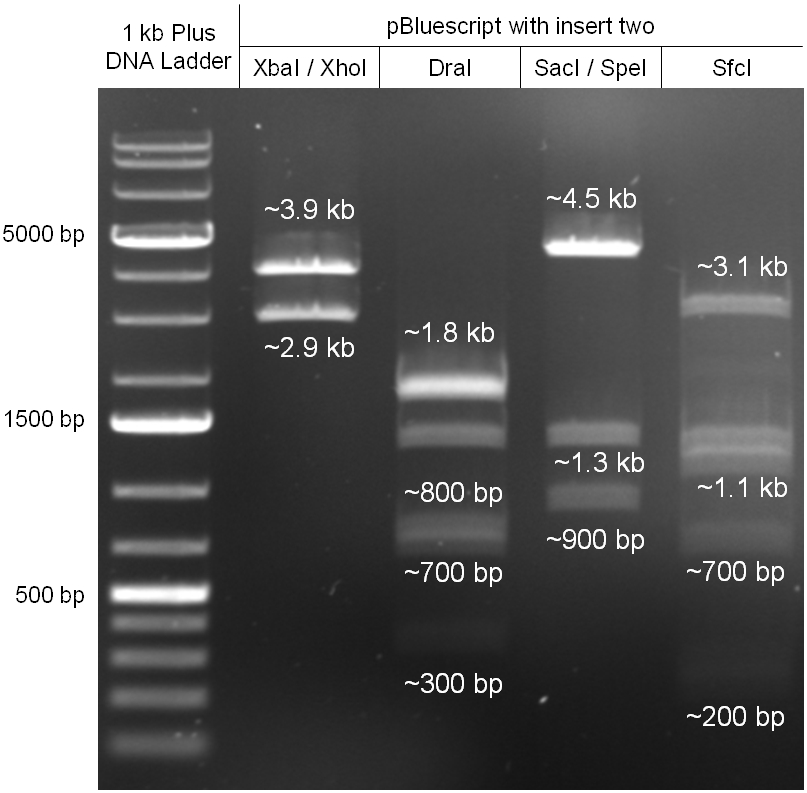
- 4 Control digestions to check wheter the ligation was successful or not
- lane0: DNA ladder mix
- lane1: XbaI/XhoI: 3,9kb / 2,9kb (NEB2, BSA)
- lane2: DraI: 1,85kb / 1,8kb / 1,35kb / 780bp / 692bp/ 339bp/ 19bp (NEB4)
- lane3: SacI/SpeI: 4.5kb / 1.3kb / 929bp / 34bp (NEB1,BSA)
- lane4: SfcI: 3.1kb / 1.3kb / 1.1kb / 678bp / 247bp / 192bp (NEB4, BSA)
- we got the correct pBluescript with the insert of phage cloning strategy two
Saturday, 10/25/08
Proceedings of phage cloning strategy one
- we got colonies on the plates with ampicillin and chloramphenicol only when our conjugated cultures were brought out, but not if T9002 or the cells harboring pUB307 and our phage were brought out separately
- this shows that our phage can be transported by conjugation
Sunday, 10/26/08
Chloramphenicol resistance cassette
- Experiment, to check until what antibiotic concentration the plasmid gives resistance
- inoculation of TOP10 and TOP10 with BBa_K150003 in LB with different Cm concentrations:
- 0 µg/ml
- 5 µg/ml
- 10 µg/ml
- 15 µg/ml
- 20 µg/ml
- 25 µg/ml
- 30 µg/ml
- 35 µg/ml
- 40 µg/ml
- 45 µg/ml
- 50 µg/ml
- 60 µg/ml
- 70 µg/ml
- 80 µg/ml
- 90 µg/ml
- 100 µg/ml
- 250 µg/ml
- 500 µg/ml
- 750 µg/ml
- 1000 µg/ml
- 5000 µg/ml
- stock solution: 100mg/ml in ethanol
- mock treated:
- 500 µg/ml
- 750 µg/ml
- 1000 µg/ml
- 5000 µg/ml
- meausurement of OD every 30min with TECAN, measurement length: 12 hours
| << Week 11 | Overview | Week 13 >> |
|---|
 "
"