Alberta NINT/16 June 2008
From 2008.igem.org
(Difference between revisions)
| (3 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
[[Team:Alberta_NINT/Notebook | < Back to notebook]] | [[Team:Alberta_NINT/Notebook | < Back to notebook]] | ||
| + | |||
| + | [[Alberta_NINT/14_June_2008 | < Previous entry]] | [[Alberta_NINT/17_June_2008 | Next entry >]] | ||
| + | |||
lab work (SD): | lab work (SD): | ||
| Line 10: | Line 13: | ||
Combined .1 and .4 to give higher concentration of DNA. | Combined .1 and .4 to give higher concentration of DNA. | ||
Ligated K102000 with I732018.1 and with I732018.2 (Negative control K102000). | Ligated K102000 with I732018.1 and with I732018.2 (Negative control K102000). | ||
| + | [[Image:NINT_SD_p41.jpg|750 px]] | ||
| + | |||
lab work (JD): | lab work (JD): | ||
| Line 15: | Line 20: | ||
Still carried out mini-prep on K102007.1 and K102007.2 from these colonies | Still carried out mini-prep on K102007.1 and K102007.2 from these colonies | ||
Sequenced K102007.1 and K102007.2 | Sequenced K102007.1 and K102007.2 | ||
| - | Since 1/100 dilution of TA0 didn't work well we want to recarry out ligations with 1/10, 1/100 and 1/1000 dilutions | + | Since 1/100 dilution of TA0 didn't work well we want to recarry out ligations with 1/10, 1/100 and |
| + | 1/1000 dilutions | ||
Performed the appropriate dilutions | Performed the appropriate dilutions | ||
Carried out ligations of K102005/B+N and 1/10, 1/100, 1/1000 dilutions of TA0/B+N | Carried out ligations of K102005/B+N and 1/10, 1/100, 1/1000 dilutions of TA0/B+N | ||
Transformed XL1-B cells | Transformed XL1-B cells | ||
Latest revision as of 22:50, 11 October 2008
< Previous entry | Next entry >
lab work (SD):
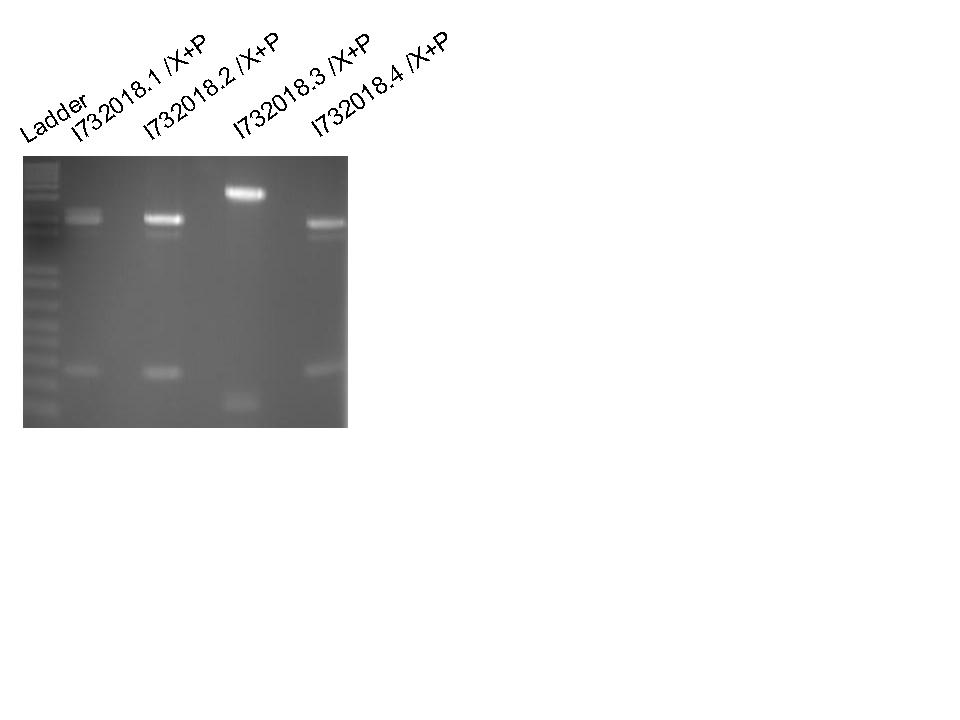
Isolated DNA (I732018) from 15/06/08 LB + amp culture tubes using QIAprep spin miniprep protocol,
washing with Buffer PB to eliminate any potential high nuclease activity and prevent loss of DNA.
Set up I732018 for sequencing.
Digested I732018 with XbaI + PstI.
Ran on 2% agarose gel (110V 50 minutes) and excised the 250 bp fragments from I732018.1, .2 and .4
Extracted DNA from gel usig QIAquick gel extraction protocol.
Combined .1 and .4 to give higher concentration of DNA.
Ligated K102000 with I732018.1 and with I732018.2 (Negative control K102000).
lab work (JD):
Only 2 colonies were found on K102007 positive and negative plates so ligation didn't work
Still carried out mini-prep on K102007.1 and K102007.2 from these colonies
Sequenced K102007.1 and K102007.2
Since 1/100 dilution of TA0 didn't work well we want to recarry out ligations with 1/10, 1/100 and
1/1000 dilutions
Performed the appropriate dilutions
Carried out ligations of K102005/B+N and 1/10, 1/100, 1/1000 dilutions of TA0/B+N
Transformed XL1-B cells
 "
"