Team:Bologna/Biosafety
From 2008.igem.org
Rita.morini (Talk | contribs) |
Rita.morini (Talk | contribs) |
||
| Line 26: | Line 26: | ||
<br> | <br> | ||
| - | [[Image:Biohazard.jpg|Biosafety| | + | [[Image:Biohazard.jpg|Biosafety|center|300px]] |
<div style="text-align:justify"> | <div style="text-align:justify"> | ||
Revision as of 13:24, 24 October 2008
| HOME | TEAM | PROJECT | MODELING | WET-LAB | SOFTWARE | SUBMITTED PARTS | BIOSAFETY AND PROTOCOLS |
|---|
Contents |
What is biosafety?
From the earliest days of microbiological research, laboratorians have recognized that acquiring infections from the agents they manipulated was a recognized occupational hazard. The most commonly-acquired lab infections were caused by bacterial agents; as microbiologists learned to culture animal viruses, they also found ways to become infected with these agents. Exposure to infectious aerosols were implicated in about eighty per cent of the reported infections.
Guidelines evolved as a means of protecting microbiological workers based on these data and an understanding of the risks associated with various manipulations of many agents transmissible by different routes. The different biosafety levels developed for microbiological and biomedical laboratories provide increasing levels of personnel and environmental protection.
There is a definite hierarchy of administrative controls that need to be in effect. Upper level management must set the general tone that safety is a high priority at their institute. Crucial to safe working conditions are the various types of specialized equipment available to serve as primary barriers between the microorganism and the laboratorian. These range from simple gloves and other personnel protective equipment to simple (sealed centrifuge heads) or complex (biosafety cabinets) containment devices.
Levels
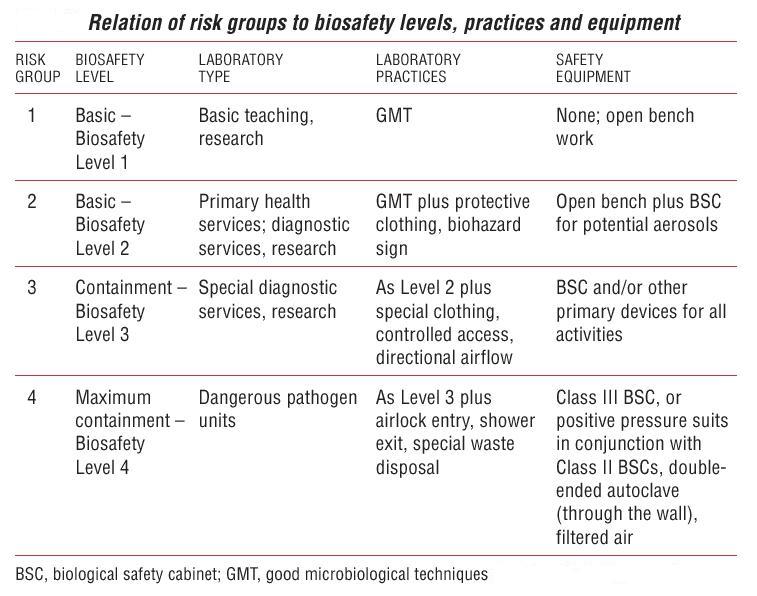
A Biosafety Level is the level of the biocontainment precautions required to isolate dangerous biological agents in an enclosed facility. The levels of containment range from the lowest biosafety level 1 to the highest at level 4.
Biosafety Level 1
This level is suitable for work involving well-characterized agents not known to consistently cause disease in healthy adult humans, and of minimal potential hazard to laboratory personnel and the environment.
It includes several kinds of bacteria and viruses including canine hepatitis, Escherichia coli, varicella (chicken pox), as well as some cell cultures and non-infectious bacteria. At this level precautions against the biohazardous materials in question are minimal, most likely involving gloves and some sort of facial protection. The laboratory is not necessarily separated from the general traffic patterns in the building. Work is generally conducted on open bench tops using standard microbiological practices. Usually, contaminated materials are left in open (but separately indicated) rubbish receptacles. Decontamination procedures for this level are similar in most respects to modern precautions against everyday microorganisms (i.e.: washing one's hands with anti-bacterial soap, washing all exposed surfaces of the lab with disinfectants, etc). In a lab environment, all materials used for cell and/or bacteria cultures are decontaminated via autoclave. Laboratory personnel have specific training in the procedures conducted in the laboratory and are supervised by a scientist with general training in microbiology or a related science.
Biosafety Level 2
This level is similar to Biosafety Level 1 and is suitable for work involving agents of moderate potential hazard to personnel and the environment. Includes various bacteria and viruses that cause only mild disease to humans, or are difficult to contract via aerosol in a lab setting, such as C. diff, hepatitis A, B, and C, influenza A, Lyme disease, dengue fever, Salmonella, mumps, Bacillus subtilis, measles, HIV, scrapie.
BSL-2 differs from BSL-1 in that:
- laboratory personnel have specific training in handling pathogenic agents and are directed by scientists with advanced training;
- access to the laboratory is limited when work is being conducted;
- extreme precautions are taken with contaminated sharp items; and
- certain procedures in which infectious aerosols or splashes may be created are conducted in biological safety cabinets or other physical containment equipment.
Biosafety Level 3
This level is applicable to clinical, diagnostic, teaching, research, or production facilities in which work is done with indigenous or exotic agents which may cause serious or potentially lethal disease as a result of exposure by the inhalation route. Includes various bacteria and viruses that can cause severe to fatal disease in humans, but for which vaccines or other treatment exist, such as anthrax, West Nile virus, Venezuelan equine encephalitis, Eastern equine encephalitis, SARS, tuberculosis, typhus, Rift Valley fever, Rocky Mountain spotted fever, yellow fever.
Laboratory personnel have specific training in handling pathogenic and potentially lethal agents, and are supervised by competent scientists who are experienced in working with these agents. This is considered a neutral or warm zone.
All procedures involving the manipulation of infectious materials are conducted within biological safety cabinets or other physical containment devices, or by personnel wearing appropriate personal protective clothing and equipment. The laboratory has special engineering and design features.
It is recognized, however, that some existing facilities may not have all the facility features recommended for Biosafety Level 3 (i.e., double-door access zone and sealed penetrations). In this circumstance, an acceptable level of safety for the conduct of routine procedures, (e.g., diagnostic procedures involving the propagation of an agent for identification, typing, susceptibility testing, etc.), may be achieved in a Biosafety Level 2 facility, providing
- the exhaust air from the laboratory room is discharged to the outdoors,
- the ventilation to the laboratory is balanced to provide directional airflow into the room,
- access to the laboratory is restricted when work is in progress, and
- the recommended Standard Microbiological Practices, Special Practices, and Safety Equipment for Biosafety Level 3 are rigorously followed.
The decision to implement this modification of Biosafety Level 3 recommendations are made only by the laboratory director.
Biosafety Level 4
This level is required for work with dangerous and exotic agents that pose a high individual risk of aerosol-transmitted laboratory infections, agents which cause severe to fatal disease in humans for which vaccines or other treatments are not available, such as Bolivian and Argentine hemorrhagic fevers, smallpox (there is a vaccine), Marburg virus, Ebola virus, Lassa fever, Crimean-Congo hemorrhagic fever, and other various hemorrhagic diseases.When dealing with biological hazards at this level the use of a Hazmat suit and a self-contained oxygen supply is mandatory. The entrance and exit of a Level Four biolab will contain multiple showers, a vacuum room, an ultraviolet light room, and other safety precautions designed to destroy all traces of the biohazard. Multiple airlocks are employed and are electronically secured to prevent both doors opening at the same time. All air and water service going to and coming from a Biosafety Level 4 lab will undergo similar decontamination procedures to eliminate the possibility of an accidental release.
Agents with a close or identical antigenic relationship to Biosafety Level 4 agents are handled at this level until sufficient data is obtained either to confirm continued work at this level, or to work with them at a lower level.
Members of the laboratory staff have specific and thorough training in handling extremely hazardous infectious agents and they understand the primary and secondary containment functions of the standard and special practices, the containment equipment, and the laboratory design characteristics. They are supervised by qualified scientists who are trained and experienced in working with these agents. Access to the laboratory is strictly controlled by the laboratory director.
The facility is either in a separate building or in a controlled area within a building, which is completely isolated from all other areas of the building. A specific facility operations manual is prepared or adopted. Building protocols for preventing contamination often uses negatively pressurized facilities, which, if compromised, would severely inhibit an outbreak of aerosol pathogens.
Within work areas of the facility, all activities are confined to Class III biological safety cabinets, or Class II biological safety cabinets used with one-piece positive pressure personnel suits ventilated by a life support system. The Biosafety Level 4 laboratory has special engineering and design features to prevent microorganisms from being disseminated into the environment. The laboratory is kept at negative air pressure, so that air flows into the room if the barrier is penetrated or breached. Furthermore, an airlock is used during personnel entry and exit.
Biosafety in our project
In order to lower the risk of causing damages at people's health and to protect the environment, our team has instituted a commission that ensures the internal and external security of the laboratory. Every protocol is characterized by a risk index that represents the level of researcher, public and environmental safety. A low index indicates a little hazard, contrarily the presence of an high index means that strict precautions need to be adopted during the execution of the protocol.
Anyway, the most dangerous practices concern utilization of Ethidium Bromide, UV rays and genetically modified bacteria.
Ethidium Bromide and UV Rays
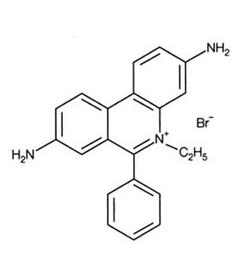
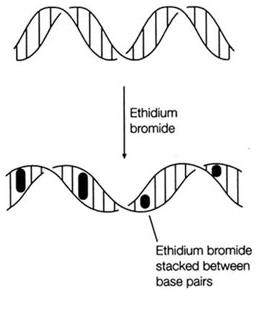
Ethidium Bromide (EtBr) is commonly used as a non-radioactive marker for identifying and visualizing nucleic acid bands in electrophoresis and in other methods of gel-based nucleic acid separation. EtBr is dark red, crystalline, non-volatile solid, moderately soluble in water, which fluoresces readily with a reddish-brown color when exposed to ultraviolet light (UV). Although it is an effective tool, its hazardous properties require special safe handling and disposal procedures.
Safety Precautions
Ethidium Bromide is a potent mutagen and is moderately toxic after an acute exposure. EtBr can be absorbed through skin, so it is important to avoid any contact with the chemical. EtBr is also an irritant to the skin, eyes, mouth and upper respiratory tract. Good work practices can help reduce hazardous exposure.
- To prevent inhalation exposure, work with EtBr powder or crystals in a fume hood, or work with premixed EtBr solutions or tablets to avoid handling the powder directly.
- To prevent skin contact when working with liquid solutions, wear nitrile gloves, a laboratory coat, and safety goggles. Change gloves frequently.
- Review an EtBr Material Safety Data Sheet (MSDS) and this EH&S fact sheet before handling the material.
- Wear eye protection and ensure that there is unobstructed access to an eyewash/shower unit in the work area.
- As with any chemical, to avoid ingestion do not eat or drink where EtBr is handled, processed, or stored.
- Always wash hands thoroughly after handling EtBr, even when gloves are used.
- Wear UV-blocking eyewear or work behind a UV shielding glass when using ultraviolet light to visualize EtBr.
Spills and Decontamination
- Use soap and water mixture (detergent solution) or 70% ethanol to wipe clean laboratory work surfaces contaminated with ethidium bromide.
- Use a UV light to survey work surfaces in the laboratory to ensure that the ethidium bromide has been removed.
Emergency Exposures
- Eye care - If EtBr comes in contact with the eyes, immediately flush them with copious amounts of cold or cool water for at least 15 minutes, preferably in emergency eyewash.
- Skin care - In the event of skin exposure, remove contaminated clothing and immediately wash the affected area with soap and copious amounts of cold or cool water for 15 minutes.
- If swallowed or inhaled - In the case of EtBr ingestion, obtain medical attention immediately. If EtBr dust is inhaled, move the victim to a source of fresh air.
Genetically modified E. coli
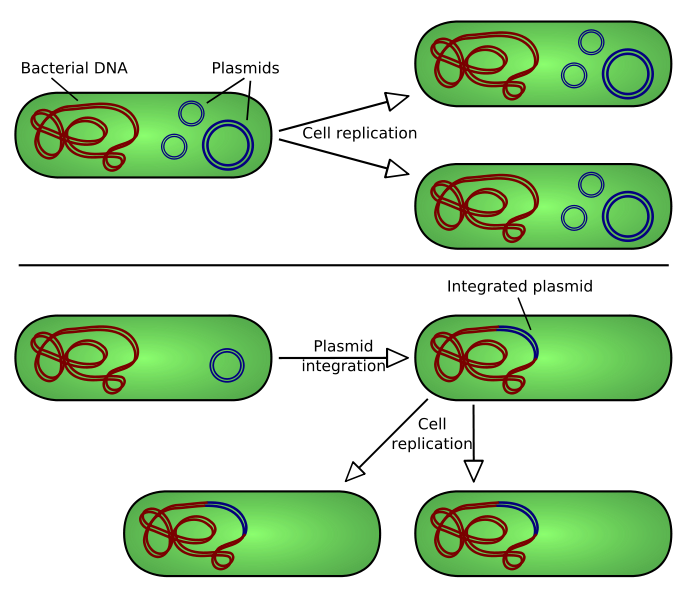
Our team uses transient transformation techniques, in which the inserted vector remains into the cell as extrachromosomic fragment. In other words it doesn't integrate itself to the cellular genome. In this case the properties inducted by the transformation endure about 72 hours.
So it's necessary to develop techniques that allow the insulation -into the cellular culture- of the cells that completed the transformation process. This function is carried out by the action of some sequences contained in the vector with the gene of interest: the selection markers. Through genetic engineering techniques, vectors are filled with a gene -anticipated by a strong promoter that encourage its transcription- that resists to a particular antibiotic. The same antibiotic is inserted in the medium: if the vector has entered it will confer the resistance to the bacteria. All the non-transformed cells will be killed.
Considering that, an eventual contamination of the environment producted by the bacteria dispersion would not involve any negative effect. This is because there are no favorable conditions for the proliferation of transformed cells. Therefore the biosafety level maintains the unitary value.
Protocols
| Protocol | Biosafety level |
| Plates preparation | |
| Amplification | |
| Transformation | |
| Inoculation | |
| Miniprep | |
| Digestion reaction | |
| Gel preparation | |
| Electrophoretic run | |
| Gel extraction | |
| Ligation reaction | |
| Chemiocompetent cells | |
| Mediums and buffers | |
| Antibiotics stocks preparation | |
| IPTG stocks preparation | |
| Fluorescence test | |
| M9 supplemented media | |
Bibliography
- Richmond, JY and RW McKinney, 1993: Biosafety in Microbiological and Biomedical Laboratories. US Department of Health and Human Services, CDC/NIH, 3rd Edition. US Government Printing Office, Washington, DC.
- US Department of Labor, Occupational Safety and Health Administration, 1991. Occupational Exposures to Bloodborne Pathogens, Final Rule. Fed. Register 56:64175-64182.
- Richmond, JY and RW McKinney, 1995: Primary Containment for Biohazards: Selection, Installation and Use of Biological Safety Cabinets. US Department of Health and Human Services, CDC/NIH.. US Government Printing Office, Washington, DC.
- Council Directive 90/679/EEC of 26 November 1990 on the protection of workers from risks related to exposure to biological agents at work, OJ No. L 374, p. 1).
- Manuel S. Barbeito; Richard H. Kruse. "A History of the American Biological Safety Association". American Biological Safety Association. Retrieved on 2008-08-14.
- "Biosafety History,Recombinant DNA Molecules,Hybrid Organisms,NIH Guidelines,Bacillus Subtilis". Molecular-Plant-Biotechnology.info. Retrieved on 2008-08-14.
Under notebook are all relevant information regarding the work done every week in the laboratory and protocols followed.
 "
"