Team:Bologna/Project
From 2008.igem.org
(→Conclusions) |
|||
| Line 35: | Line 35: | ||
| - | + | [https://2008.igem.org/Team:Bologna/Project ''Up''] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
= Results = | = Results = | ||
| + | [https://2008.igem.org/Team:Bologna/Project ''Up''] | ||
= Conclusions = | = Conclusions = | ||
| Line 60: | Line 56: | ||
* We want even to mention the courtesy of the [[Team:Valencia|Valencia]] iGEM Team, that have advised us about the critical use of GFP and RFP at the same time. | * We want even to mention the courtesy of the [[Team:Valencia|Valencia]] iGEM Team, that have advised us about the critical use of GFP and RFP at the same time. | ||
| - | |||
| - | |||
| + | [https://2008.igem.org/Team:Bologna/Project ''Up''] | ||
= Concluding the iGEM 2007 Project = | = Concluding the iGEM 2007 Project = | ||
Revision as of 11:08, 29 October 2008
| HOME | PROJECT | TEAM | SOFTWARE | MODELING | WET LAB | LAB-BOOK | SUBMITTED PARTS | BIOSAFETY AND PROTOCOLS |
|---|
Contents |
Ecoli.PROM: an Erasable and Programmable Genetic Memory in E. coli
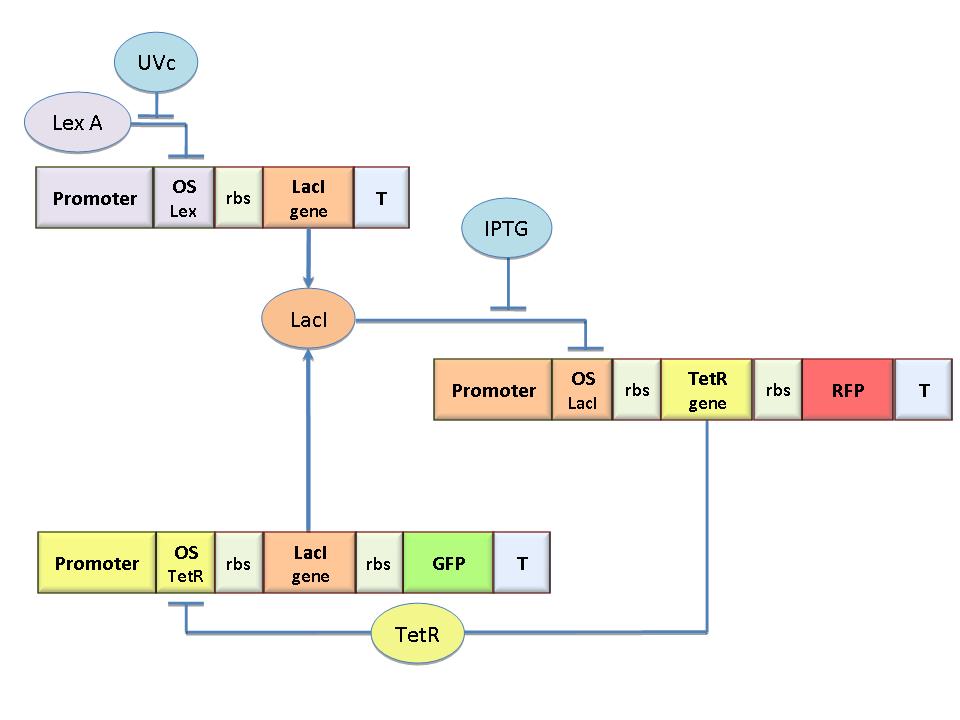
The specific goal of our project was to design a bacterial reprogrammable memory, i.e. colonies of genetically engineered E. coli immobilized in solid medium where they work as an array of binary memory cells.
To engineer the bacteria we designed a modular genetic Flip-Flop composed of two parts (Figure 1): a binary memory block and an induction block, sensitive to UV radiation, to set LacI ON. UV has been chosen to have a fine spatial selectivity in programming the memory cells, whereas IPTG should be used to reset the entire memory (TetR ON).
The core elements of the genetic memory are two mutually regulated promoters, each designed as indipendent operator sites flanking a constitutive promoter. In this way, the promoter transcriptional strength and the repressor binding affinity can be independently fixed. To this aim we designed operator libraries for LacI, TetR, Lambda and LexA repressors, cloning them in the BioBrick format for their standard assembly. This allowed us to design and assemble three different circuits, where the BBa_J23118 constitutive promoter was cloned with the Lac operator 1, the Lac operator 2 and the symmetric one from the Lac operator library. Moreover, LacI was cloned downstream of the promoter- operator sequence and the GFP was chosen as the reporter. Thus, promoter activation was under the control of the LacI repressor, and each of the circuits was expected to yield a specific promoter repression/activation profile depending on the characteristic operator- repressor binding affinity. The model-based analysis of the circuit response was used for the Ki transcription index determination and the design of the desired promoter/ operator couple needed to achieve bistability.
Results
Conclusions
This will allow the rational design of regulated promoter elements that are still lacking in the Registry.
This approach has been applied in the building of our UV-programmable memory, and we expect it to be a general benefit in a larger number of applications in Synthetic Biology.
Collaboration with other iGEM Teams 2008
- After the last year competition, at the beginning of the 2008, we decided to get a new team started for iGEM2008 competition. In April, we got in contact with Prof. Paolo Magni, who wanted to start a new team in Pavia for iGEM2008. So, in order to share experiences and ideas about iGEM, and to show him what kind of wet lab resources are necessary to develop a Synthetic Biology project, we met at the Cellular and Molecular Engineering Laboratory of the University of Bologna- Cesena Campus. After this first meeting, there have been other chances to meet during the summer. In particular, several conference calls were organized and two meetings were scheduled in Pisa and Bressanone (Italy). It was fundamental to compare lab protocols and techniques to help each other avoiding mistakes and speeding up project progress. The main topics of our discussion were the optimization of plasmid resuspension and ligation reaction steps as well as how to measure fluorescence. Finally, before DNA Repository quality control publication on the Registry web site, we cross-checked some parts that showed problems after DNA transformation. Problems had been confirmed by quality control results (parts' sequences classified as "inconsistent").
- We want even to mention the courtesy of the Valencia iGEM Team, that have advised us about the critical use of GFP and RFP at the same time.
Concluding the iGEM 2007 Project
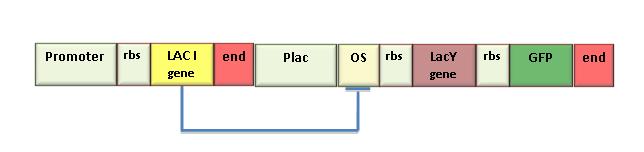
In the iGEM 2007 we used the LacY gene (BBa_J2210) to design the genetic Schmitt Trigger. Since this part was not working well, we sent it to be sequenced and found that it contained a 35 bp insertion upstream the endogenous LacY gene sequence. This insertion probably caused a frame- shift in protein translation, making the gene ineffective. So, we amplified the right gene sequence and put it in the BioBrick format. Successive sequencing confirmed the right assembling of this part. We also measured IPTG-induced fluorescence in the genetic Schmitt Trigger (see Figure) and we assessed the correct function of the new LacY part. To contribute to registry’s improvement we decided to send this new part to the Registry (K0790015).
 "
"