Team:BrownTwo/Limiter/network
From 2008.igem.org
The Genetic Limiter
Our limiter circuit is an example of a complex device built from smaller parts and circuits in the mode of the synthetic biologist. To push the limits of synthetic biology beyond the conventional bacterial chassis, we have elected to build our device in Saccharomyces cerevisiae, an oft-studied model eukaryote that has heretofore been quite negelected in iGEM competition. Leading to the limiter
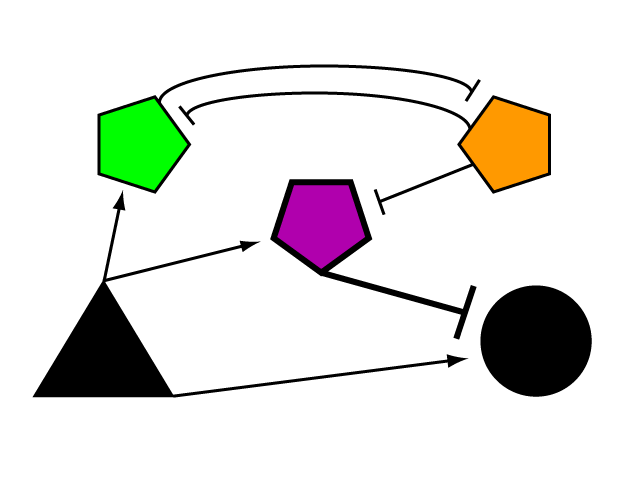
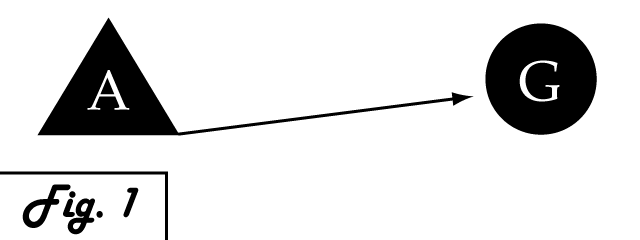
Consider a gene of interest (G) for which one inducing transcription factor (A) is known (Fig. 1). In the presence of unnaturally-heightened levels of inducer A, our gene will experience excessive activation. A logical method for addressing this problem would be to introduce an inhibitor for the gene of interest to reduce its activity. Traditional interventions either directly block the activity of gene G, thus causing interference on the level of protein interactions, or arrest protein synthesis of gene G's encoded gene product by hindering mechanisms of transcription and translation. One concern that arises with the use of a chemical input is that, especially in the case of multi-cellular organisms, this often has diffuse, unspecified, and often undesirable effects. Drugs that are administered to biological systems in a therapeutic context particularly require an understanding of the intricate balance between efficacy and toxicity. Although the application of a genetically-based inhibitor is often more difficult, such genetic parts afford a great deal of specificity to the system. Because they are embedded into the cellular program in order to affect it their activity can be significantly more pronounced.
If we were to introduce a repressor construct driven by a constitutive promoter, one might face the issue that continued expression of inducer A would surpass the repressor's ability to oppose gene G's activation. Thus, instead of a constitutive promoter, one could ensure that the repressor for the gene of interest is under the control of a promoter that also takes the same inducer A as an input. This means that the amount of repressor in the system scales with the amount of inducer. However, the introduction of a single repressor construct in this fashion will also result in passively lowering the level of gene expression for any given level of inducer. This means that G will be present in lower than normal quantities for normal amounts of inducer.
To prevent such unwanted interference of endogenous transcription, one could incorporate a second repressor to inhibit the repressor. Of course, this system of repressors would need a way of detecting At this point, it is important to turn to a concept from electrical engineering for inspiration. The Bistable SwitchA bistable switch is a simple circuit in which, depending upon the nature or quantity of the input, two stable outcomes are possible. In one case, would allow the system to alternate between two modes of activity in response to varying levels of input. Dr. James Collins from Boston University developed a bistable switch capable of switching between two genetic outputs based upon the inputs to the system. Driven by the desire to create a synthetic gene network that could act similarly to endogenous regulatory pathways, we developed a design for a system that had the ability to regulate We began by developing a layout for
Diagram our network and explain why we laid it out this way.
|
 "
"