Team:EPF-Lausanne/2-step PCR
From 2008.igem.org
| Home | The Team | The Project | Parts | 2-step PCR | Microfluidics | Modeling | Notebook |
|---|
2-steps PCR
The 2-steps PCR is a technique used to add small DNA sequences to a PCR product. In the IGEM context, this can be used as an alternative to classical cloning: instead of digesting and ligating each biobricks, small part can be directly added by PCR, saving a lot of time.
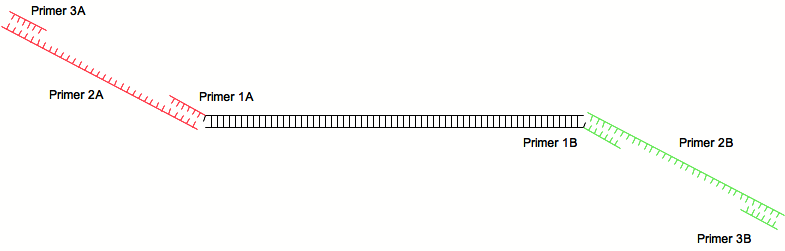
The 2-step PCR requires three pairs of primer and three PCR runs:
A first pair is used to amplify a sequence of interest (for example an ORF) and provides annealing sites for the second pair of primers:
The second pairs anneals with the first and contains the sequence wanted to be add to the PCR product. The third pairs of primer amplify the initial PCR product with the added sequence.
Results:
During our project, we used this technique to clone the ORF of the gene luxI (the corresponding Biobrick, F1610, seemed inconsistent). The first PCR was performed on a plasmid to amplify the ORF of LuxI. During the second step, a RBS and the prefix were added upstream, as a terminator and the suffix were added downstream. The result of the this 2-step PCR was digested and inserted in pSB1A, giving the Biobrick BBa_K092400)
Time saved
The major advantage of this technique is time needed to create clone. A RBS, a promoter and a terminator can be added to an ORF in a few hours. The result of this 2-step PCR can be digest and ligated into a vector. In total, this operation needs approximately 30 hours. In comparison, creating the same clone using classical cloning would need six days.
Time table
 "
"