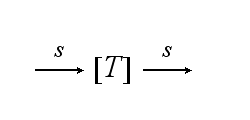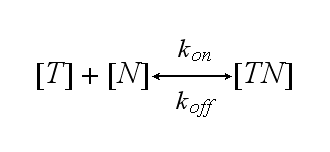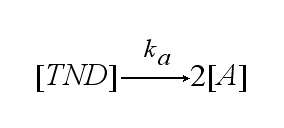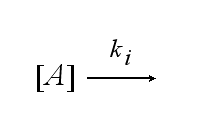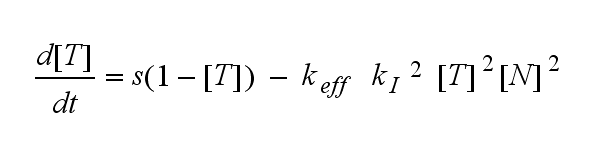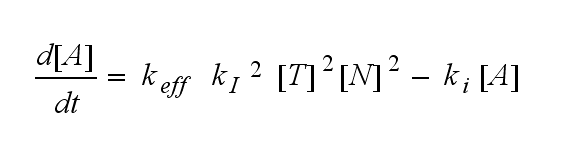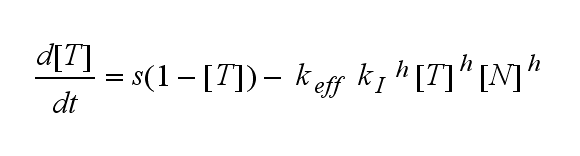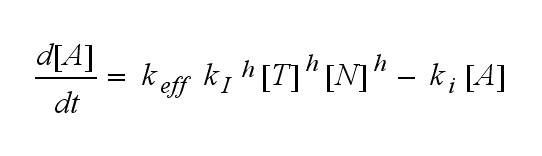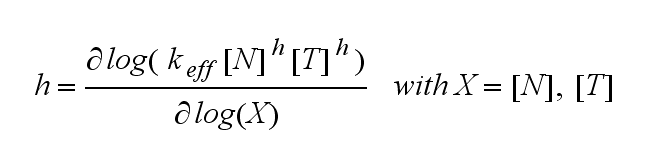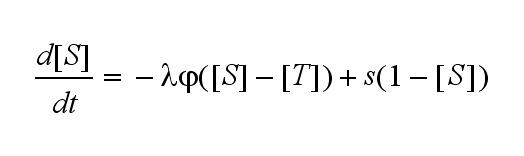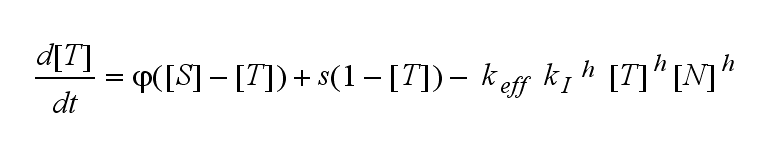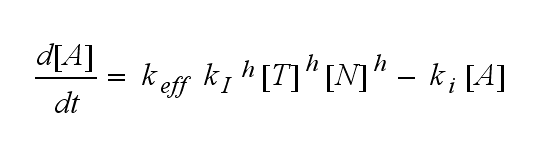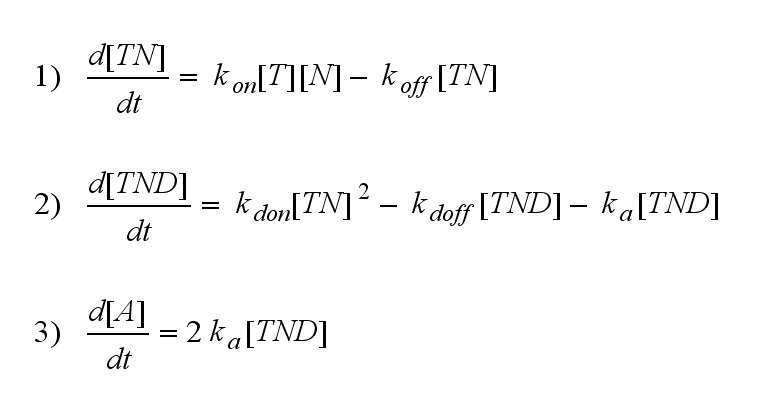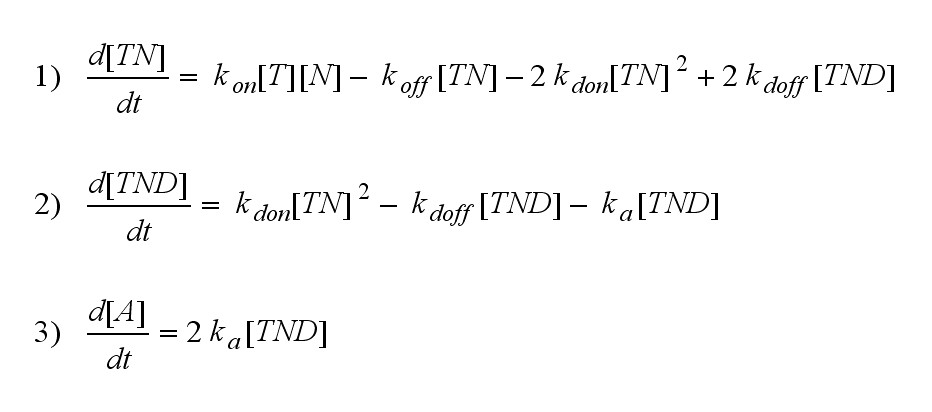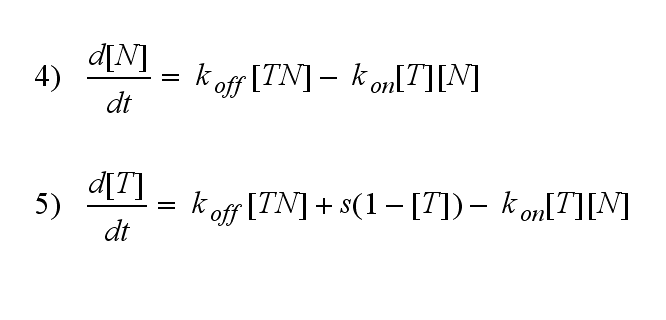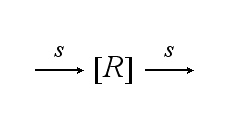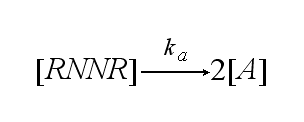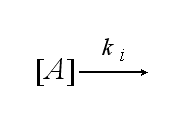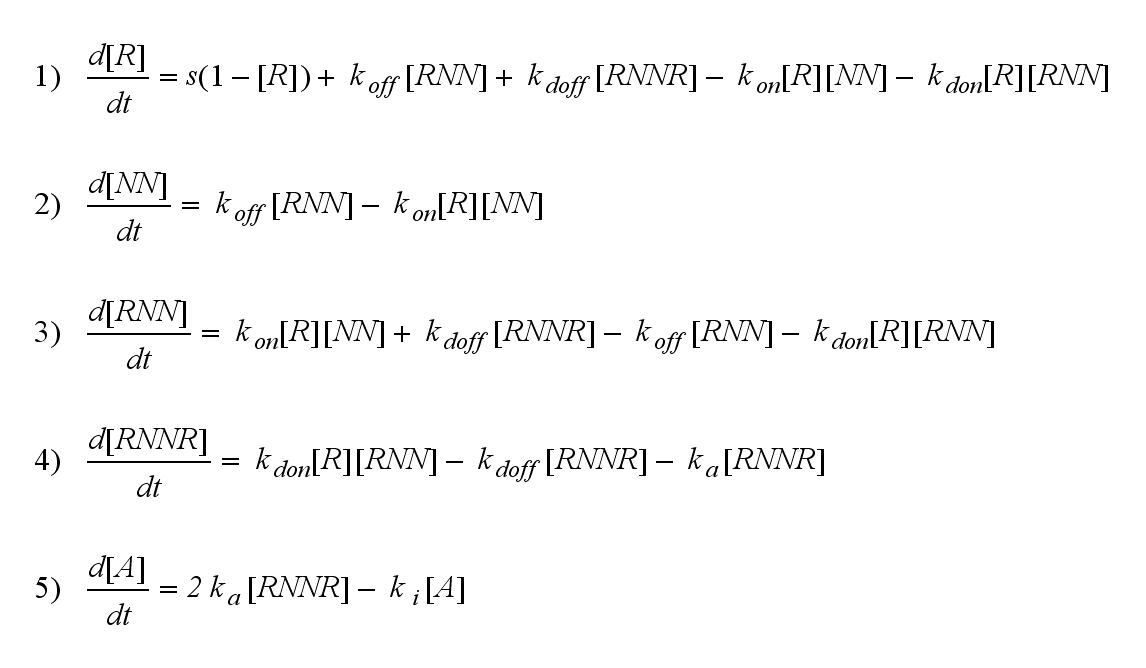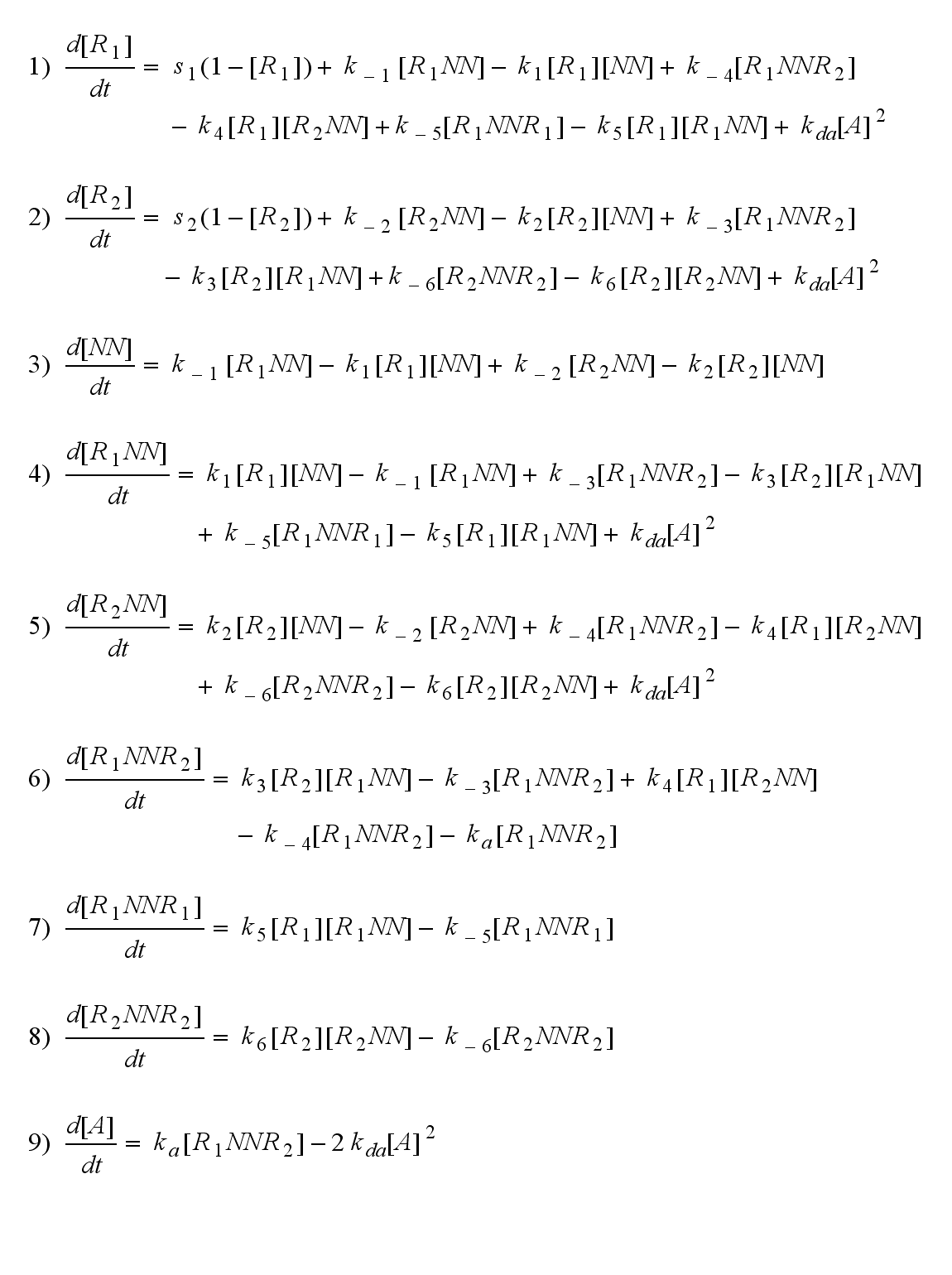Team:Freiburg/Modeling
From 2008.igem.org
m |
|||
| (81 intermediate revisions not shown) | |||
| Line 6: | Line 6: | ||
__NOTOC__ | __NOTOC__ | ||
<h1>Introduction</h1> | <h1>Introduction</h1> | ||
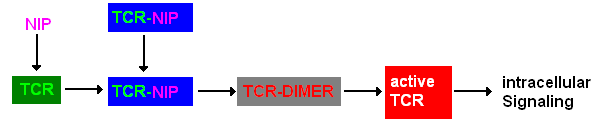
| - | + | Dimerization of the extracellular receptor domains is an important necessity for the functionality of our Modular Synthetic Receptor System. Presenting the system a stimulus in the form of spatial arranged ligands results in dimerization of the extracellular domains and thus the corresponding intracellular parts such as the split lactamase halves or split fluorescent proteins complement to a measurable output. To analyze the functionality due to dimerization, first two receptor dimerization models (one T cell receptor model and one general receptor model) are introduced and discussed and then a proper [[Team:Freiburg/Modeling#Modular Synthetic Receptor System model|model for the Modular Synthetic Receptor System]] is constructed. | |
<br><br> | <br><br> | ||
<font style="color:#333366; font-style:italic;"> | <font style="color:#333366; font-style:italic;"> | ||
| - | As | + | As given by the diversity of our transmembrane fusion proteins, some of the constructs can bind fluorescein as ligand as well. As the model for the Modular Synthetic Receptor System is applicable for all our constructs, the ligand usage is extended with fluorescein. So either NIP or fluorescein can function as ligand, dependent on the choice of constructs for implementation. |
</font> | </font> | ||
| Line 17: | Line 17: | ||
<h1>T cell receptor dimerization model I </h1> | <h1>T cell receptor dimerization model I </h1> | ||
| + | [[media:Freiburg2008_M1_Basic.m|''matlab m-file'']]<br> | ||
<br> | <br> | ||
| - | T cells are a special type of white blood cells (lymphocytes) and play a central role in cell-mediated immunity. They carry special receptors, so called T cell receptors (TCR) on their membrane. One part of the mechanisms to activate a TCR and thus to activate a T | + | T cells are a special type of white blood cells (lymphocytes) and play a central role in cell-mediated immunity. They carry special receptors, so called T cell receptors (TCR) on their membrane. One part of the mechanisms to activate a TCR and thus to activate a T cell is the binding of a ligand, also called antigen, to the TCR. As research showed, one single ligand-TCR complex does not lead to a T cell response yet, as at least two ligand-TCR complexes and their dimerization seem to be required for proper T cell activation (Schamel, 2006; Bachmann 1999). |
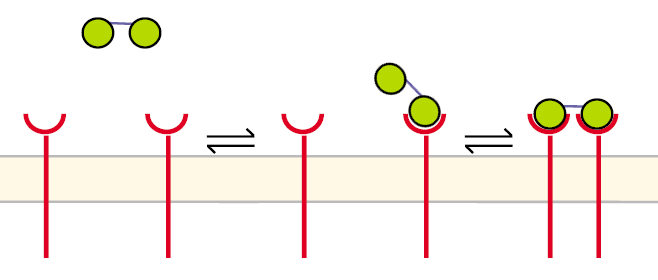
| - | In our case the ligands are nitro-iodo-phenol (NIP) molecules attached to a DNA-Origami structure at a (variable) distance of ~6nm to each other. These NIPs are recognized and bound by the TCRs. As the distance is small enough for two TCRs to approach very closely when each of them binds a NIP, they can dimerize. | + | In our case the ligands are nitro-iodo-phenol (NIP) molecules attached to a [[DNA-Origami|DNA-Origami]] structure at a (variable) distance of ~6nm to each other. These NIPs are recognized and bound by the TCRs. As the distance is small enough for two TCRs to approach very closely when each of them binds a NIP, they can dimerize. |
<br><br> | <br><br> | ||
<h3>Extracellular signaling </h3> | <h3>Extracellular signaling </h3> | ||
| - | A simplified pathway shows the extracellular sequence of TCR activation. After the NIP binding two complexes come together and form a dimer which then leads to | + | A simplified pathway shows the extracellular sequence of TCR activation. After the NIP binding two complexes come together and form a dimer which then leads to activation of the TCR and further intracellular signaling and T cell activation. |
<table> | <table> | ||
<tr> | <tr> | ||
| Line 55: | Line 56: | ||
<br><br> | <br><br> | ||
| - | After activation, the TCR is | + | After activation, the TCR is internalized with rate '''k'''<sub>i</sub> and does not take part anymore in the extracellular signaling : |
<br> | <br> | ||
[[Image:Freiburg2008_RKM1int.png|150px]] | [[Image:Freiburg2008_RKM1int.png|150px]] | ||
| Line 67: | Line 68: | ||
<br><br><br> | <br><br><br> | ||
<h3>TCR activity for a set of parameters</h3> | <h3>TCR activity for a set of parameters</h3> | ||
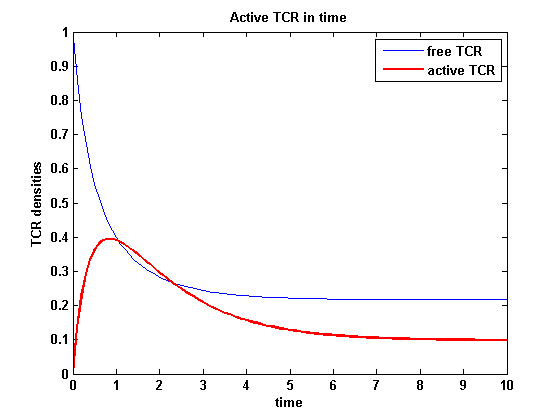
| - | The two ODEs above of this first basic model for a set of parameters are solved numerically. They reveal the | + | The two ODEs above of this first basic model for a set of parameters are solved numerically. They reveal the time course of the TCR activity and the time course of the unbound TCR aswell. |
<table> | <table> | ||
<tr> | <tr> | ||
| Line 87: | Line 88: | ||
TA0 = 0; % active TCR density | TA0 = 0; % active TCR density | ||
</font> | </font> | ||
| - | <td> | + | </td> |
| - | <tr> | + | </tr> |
</table> | </table> | ||
| - | The activity has a maximum a short time after NIP presentation and decreases as the active TCRs are internalized and as the free membrane TCR amount is decreasing. It is remarkable that the response never decreases to complete zero, even for very high internalization rates and in a long | + | The activity has a maximum a short time after NIP presentation and decreases as the active TCRs are internalized and as the free membrane TCR amount is decreasing. It is remarkable that the response never decreases to complete zero, even for very high internalization rates and in a long time course. |
<br><br> | <br><br> | ||
| - | <h2>Extensions: | + | <h2>Extensions: Ultrasensitivity and biphasic kinetics</h2> |
| + | [[media:Freiburg2008_M1_Xten.m|''matlab m-file'']]<br> | ||
<h3>Ultrasensitivity</h3> | <h3>Ultrasensitivity</h3> | ||
The kinetics of the TCR activation can be generalised by substituting the second order kinetic of the ligand N and the receptor T by a parameter h, which then represents the kinetic order of the system.<br> | The kinetics of the TCR activation can be generalised by substituting the second order kinetic of the ligand N and the receptor T by a parameter h, which then represents the kinetic order of the system.<br> | ||
| Line 104: | Line 106: | ||
<br><br> | <br><br> | ||
<h3>Biphasic kinetics and [[Team:Freiburg/Modeling/Parameter analysis|parameter analysis]]</h3> | <h3>Biphasic kinetics and [[Team:Freiburg/Modeling/Parameter analysis|parameter analysis]]</h3> | ||
| - | Not all TCRs on a T cell membrane can be recruited to NIP binding as a cell´s membrane contains several transmembrane proteins whose size can avoid a TCR-NIP formation when they surround a TCR and make the approaching of the NIP to the binding side of the TCR impossible. Considerating this spatial barriers leads to the idea of a TCR which can switch between two states, one binding state and one non-binding state. Hence a introduction of two different pools of TCRs into the model is appropriate. If a TCR is not available for a NIP molecule, thus it is in the non-binding state,it belongs to the so called '''spare''' pool, whereby TCRs belonging to the so called '''interface''' pool are in the binding state and can be accessed by the NIP molecule. Moreover the spare pool is in dynamical exchange with the interface pool, so non-binding | + | Not all TCRs on a T cell membrane can be recruited to NIP binding as a cell´s membrane contains several transmembrane proteins whose size can avoid a TCR-NIP formation when they surround a TCR and make the approaching of the NIP to the binding side of the TCR impossible. Considerating this spatial barriers leads to the idea of a TCR which can switch between two states, one binding state and one non-binding state. Hence a introduction of two different pools of TCRs into the model is appropriate. If a TCR is not available for a NIP molecule, thus it is in the non-binding state, it belongs to the so called '''spare''' pool, whereby TCRs belonging to the so called '''interface''' pool are in the binding state and can be accessed by the NIP molecule. Moreover the spare pool is in dynamical exchange with the interface pool, so non-binding TCRs can become binding TCRs. This exchange is regulated through the parameter '''λ''', a ratio between the spare and the interface pool and '''φ''', the exchange rate constant. '''S''' is the spare pool TCR density and '''T''' the TCR density of the interface pool. '''A''' represents the active TCR density. So the full model I equations are:<br> |
Spare pool: | Spare pool: | ||
[[Image:Freiburg2008_M1odeT_spare.png|380px]]<br> | [[Image:Freiburg2008_M1odeT_spare.png|380px]]<br> | ||
| Line 131: | Line 133: | ||
ki = 1; % internalization rate | ki = 1; % internalization rate | ||
N = 0.2; % NIP amount | N = 0.2; % NIP amount | ||
| - | h = 2; % | + | h = 2; % kinetic order |
</font> | </font> | ||
initial integration conditions: | initial integration conditions: | ||
| Line 142: | Line 144: | ||
</tr> | </tr> | ||
</table> | </table> | ||
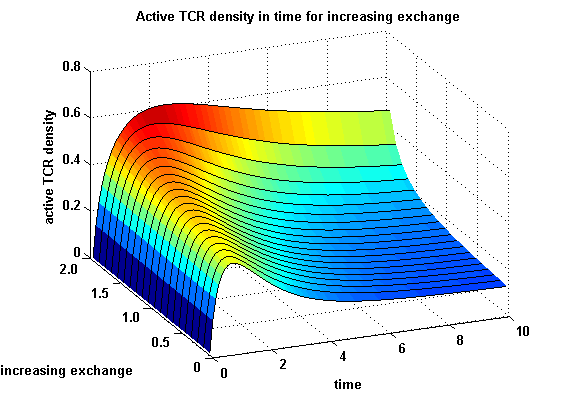
| - | The x-axis represents the | + | The x-axis represents the time course of the activity, the y-axis represents both parameters φ ( = y) and λ ( = 2 - y). So each black line in the plot is a time course of the TCR activity for a different φ and λ. The z-axis is the response intensity. With increasing exchange between the interface and spare pool, more TCRs switch to the binding state, hence more TCRs can bind NIP. As a consequence the active TCR density is higher than for a low exchange. <br> |
| - | <h2> | + | <h2>Correction terms</h2> |
| + | [[media:Freiburg2008_M1_Corrected.m|''matlab m-file'']]<br><br> | ||
Regarding the reaction kinetics and considering the ODEs as a model for TCR dimerization (Bachmann, 1999) led to the realization of an error in the mentioned publication. The ODEs evolved from the reaction kinetics are '''not''' : | Regarding the reaction kinetics and considering the ODEs as a model for TCR dimerization (Bachmann, 1999) led to the realization of an error in the mentioned publication. The ODEs evolved from the reaction kinetics are '''not''' : | ||
<br> | <br> | ||
| Line 161: | Line 164: | ||
<br><br><br><br><br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br><br><br><br><br> | ||
The NIP density is not a static value anymore but time dependent also. | The NIP density is not a static value anymore but time dependent also. | ||
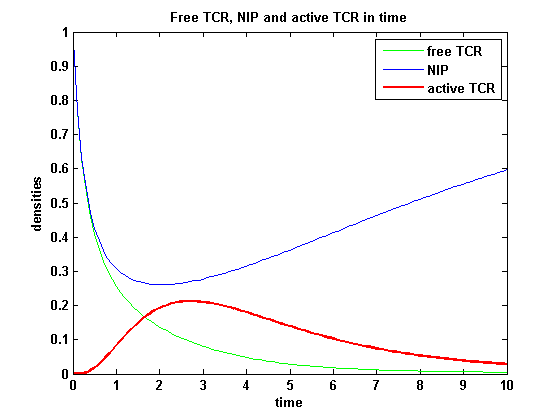
| - | On that account the last five equations are the corrected model equations. Solving this five equations numerically for a set of parameters gives a similar | + | On that account the last five equations are the corrected model equations. Solving this five equations numerically for a set of parameters gives a similar time course of the active TCR density but the response strength is lower than in [[Team:Freiburg/Modeling#ODEs derived from the kinetics (Details)|the original version]], although the initial NIP amount is five times higher: |
<table> | <table> | ||
<tr> | <tr> | ||
<td> | <td> | ||
| - | [[image:Freiburg2008_NIP_T_TA.png|thumb|left|400px|'''Figure 4:''' | + | [[image:Freiburg2008_NIP_T_TA.png|thumb|left|400px|'''Figure 4:''' Time course of NIP, free TCR and active TCR for a set of parameters]] |
</td> | </td> | ||
<td> | <td> | ||
| Line 189: | Line 192: | ||
</tr> | </tr> | ||
</table> | </table> | ||
| - | After NIP addition the TCR activity rises to a maximum and decreases as active receptors are internalized and the NIP amount is used up. The free receptor density recovers due to permanent expression of new receptors which are | + | After NIP addition the TCR activity rises to a maximum and decreases as active receptors are internalized and the NIP amount is used up. The free receptor density recovers due to permanent expression of new receptors which are built into the membrane. |
<br> | <br> | ||
<h1>Receptor dimerization model II</h1> | <h1>Receptor dimerization model II</h1> | ||
| - | + | [[media:Freiburg2008_M_2.m|''matlab m-file'']]<br><br> | |
| - | A receptor dimerization can proceed in a different way aswell, especially when two | + | A receptor dimerization can proceed in a different way aswell, especially when two ligand molecules are linked to a structure like our DNA-Origami: |
<table> | <table> | ||
<tr> | <tr> | ||
| Line 236: | Line 239: | ||
<br> | <br> | ||
<h3>ODE derivation </h3> | <h3>ODE derivation </h3> | ||
| - | The ODEs | + | The ODEs are derived from the reaction kinetics: <br> |
[[Image:Freiburg2008_ODEs_M2.png|800px]] | [[Image:Freiburg2008_ODEs_M2.png|800px]] | ||
<br> | <br> | ||
| Line 271: | Line 274: | ||
<br> | <br> | ||
<h1>Discussion of model I and II</h1> | <h1>Discussion of model I and II</h1> | ||
| - | For | + | For comparison, the ODEs of the corrected model 1 and model 2 are taken. |
| - | The receptor NIP binding mechanism differs in the two models. Model I assumes the production of two independent receptor-NIP complexes which can dimerize after formation. In model II, dimerization happens after one of two receptors connects to the bivalent ligand | + | The receptor NIP binding mechanism differs in the two models. Model I assumes the production of two independent receptor-NIP complexes which can dimerize after formation. In model II, dimerization happens after one of two receptors connects to the bivalent ligand . Setting several conditions unravels the consequences of this differences for the active receptor, the dimer and the receptor recovery. Different binding rates, dimerization rates and ligand amounts are used in the solutions of the model I ODEs and model II ODEs. |
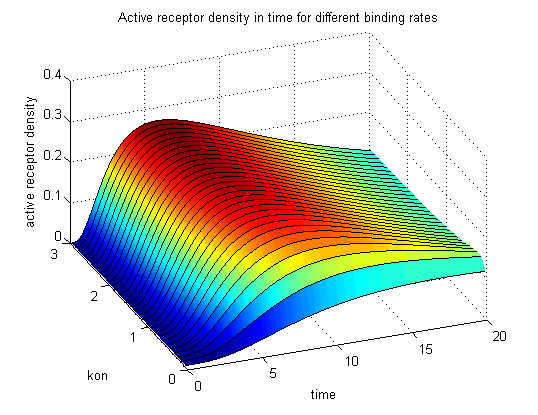
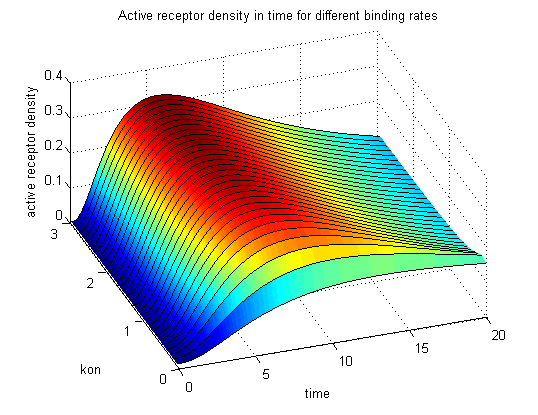
| - | <h3> | + | <h3>Receptor densities dependent on binding rate k<sub>on</sub></h3> |
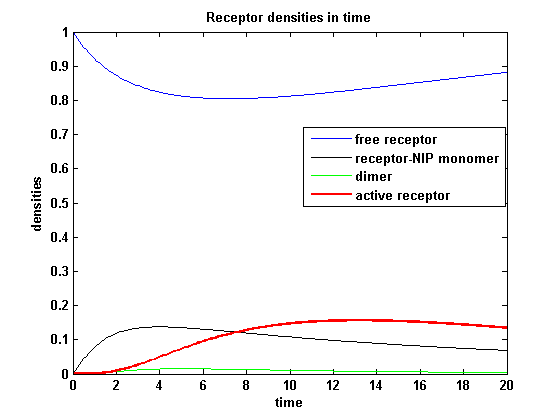
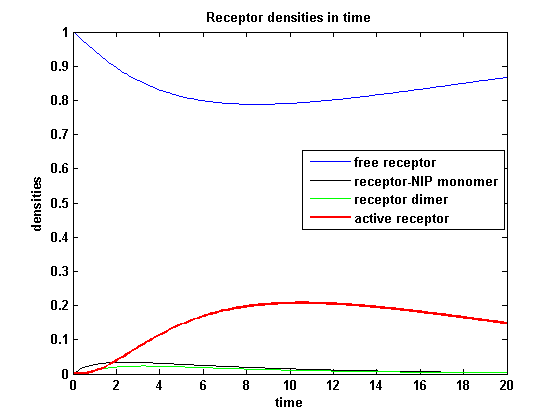
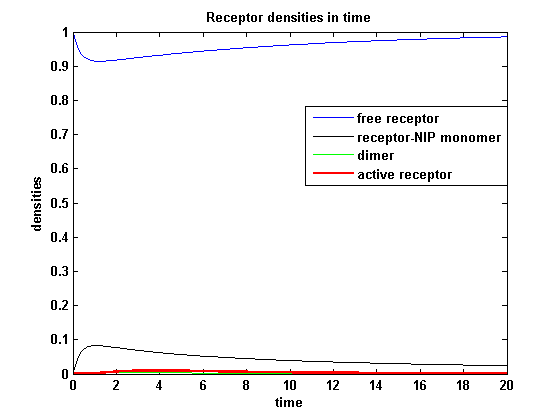
The two model ODEs are solved for the mentioned parameters below whereby the binding rate k<sub>on</sub> was variated. | The two model ODEs are solved for the mentioned parameters below whereby the binding rate k<sub>on</sub> was variated. | ||
So the course in time for the free receptor, the monomeric receptor-NIP complex, the dimer and the active receptor is plotted:<br> | So the course in time for the free receptor, the monomeric receptor-NIP complex, the dimer and the active receptor is plotted:<br> | ||
| Line 324: | Line 327: | ||
</table> | </table> | ||
<br> | <br> | ||
| - | The production of receptor-NIP complexes is lower in model 2 than in model 1. Increasing k<sub>on</sub> leads to an increase in the formation of receptor NIP-complexes thus to a higher dimerization and receptor activation in both models. In model 1 receptor-NIP densities | + | The production of receptor-NIP complexes is lower in model 2 than in model 1. Increasing k<sub>on</sub> leads to an increase in the formation of receptor NIP-complexes thus to a higher dimerization and receptor activation in both models. In model 1 receptor-NIP densities have approximately the same maxima as the active receptor densities and in model 2, receptor-NIP densities are much lower than active receptor densities. Active receptor densities react in both models similar to k<sub>on</sub> variations for the used parameters. |
<br> | <br> | ||
<br> | <br> | ||
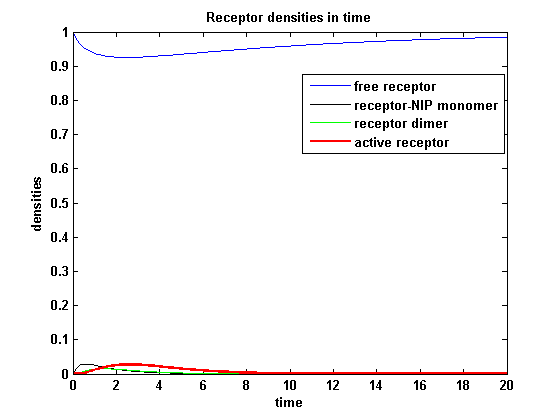
| - | <h3> | + | <h3>Receptor densities dependent on dimerization rate k<sub>don</sub></h3> |
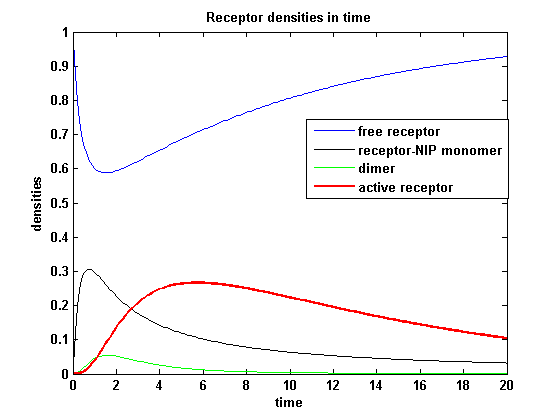
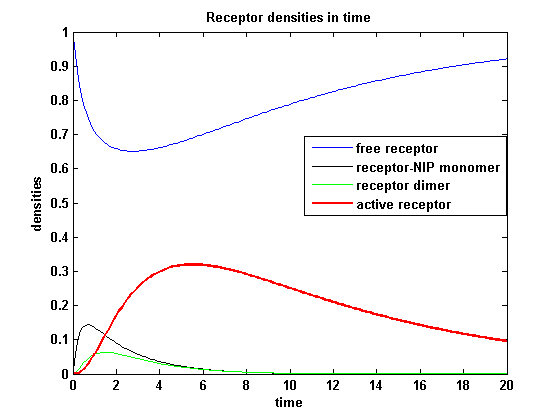
Applying different k<sub>don</sub> leads to the following result: | Applying different k<sub>don</sub> leads to the following result: | ||
<table> | <table> | ||
| Line 377: | Line 380: | ||
</table> | </table> | ||
<br> | <br> | ||
| - | A low k<sub>don</sub> gives in both models a high receptor-NIP monomer density as few of this complexes are recruited to build dimers and remain receptor-NIP monomers. This density decreases for high k<sub>don</sub> due to a higher | + | A low k<sub>don</sub> gives in both models a high receptor-NIP monomer density as few of this complexes are recruited to build dimers and remain receptor-NIP monomers. This density decreases for high k<sub>don</sub> due to a higher dimerization and receptor activity. k<sub>don</sub> variations give similar changes in receptor activity in both models. |
<br><br> | <br><br> | ||
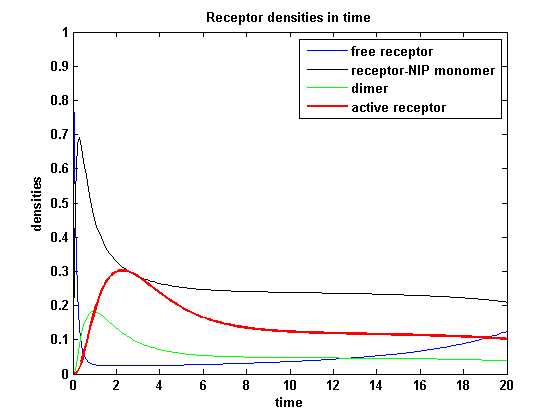
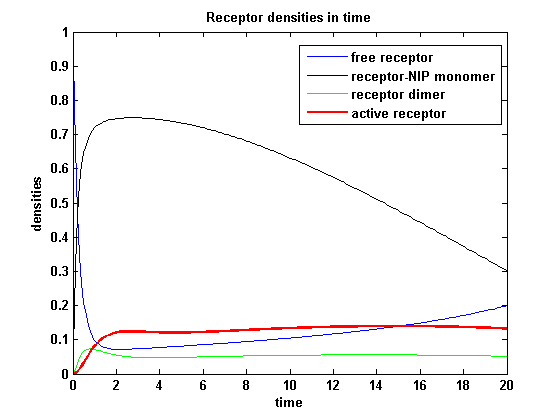
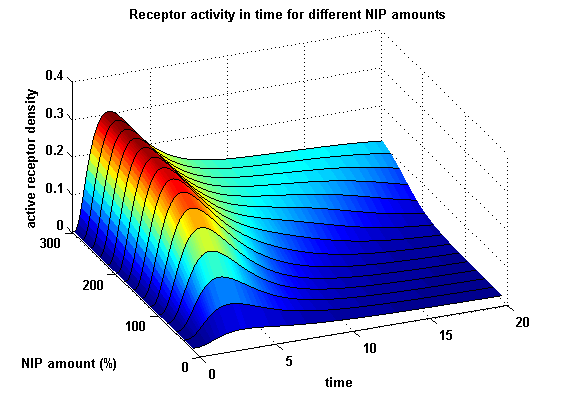
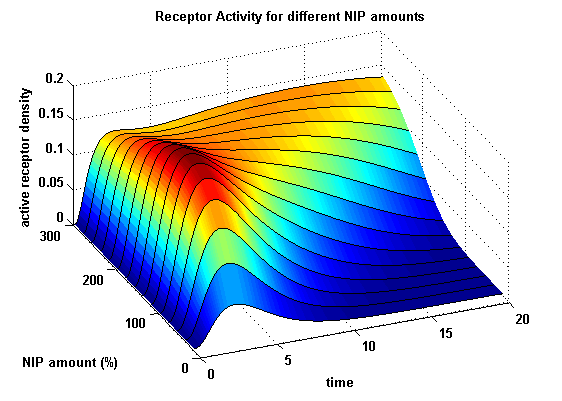
| - | <h3> | + | <h3>Receptor densities dependent on NIP amount</h3> |
| - | The use of different amounts of ligand | + | The use of different amounts of ligand is maybe the most important influence on the receptor activation. As the ligand is the stimulus in order to activate our system, it´s amount will determine strongly the activity of the receptor. So different amounts of NIP are used and a higher internalization rate k<sub>i</sub> is used aswell. |
<table> | <table> | ||
<tr> | <tr> | ||
| Line 388: | Line 391: | ||
<font color="#336600"> | <font color="#336600"> | ||
s = 0.1; % turnover rate | s = 0.1; % turnover rate | ||
| - | kon : | + | kon : 3 % receptor-NIP binding rate |
koff = 0.1; % receptor-NIP dissociating rate | koff = 0.1; % receptor-NIP dissociating rate | ||
kdon = 1; % receptor-NIP dimerization rate | kdon = 1; % receptor-NIP dimerization rate | ||
| Line 431: | Line 434: | ||
</table> | </table> | ||
<br><br> | <br><br> | ||
| - | As k<sub>i</sub> | + | As k<sub>i</sub>=0.8, the receptor activity is decreasing due to a fast internalization of active receptors. For a little stimulus the active receptor activity is almost zero. Increasing the amount of NIP leads in both models to an increase of the active receptor density. It saturates for increasing amounts of NIP in model 1. In model 2 the active receptor density increases for increasing NIP amounts, but when the NIP amount reaches a certain level, it starts to decrease as too much receptor-NIP complexes are formed, leaving few receptors for dimerization. Aswell there can be observed a late activation for high NIP amounts in model 2. |
<br> | <br> | ||
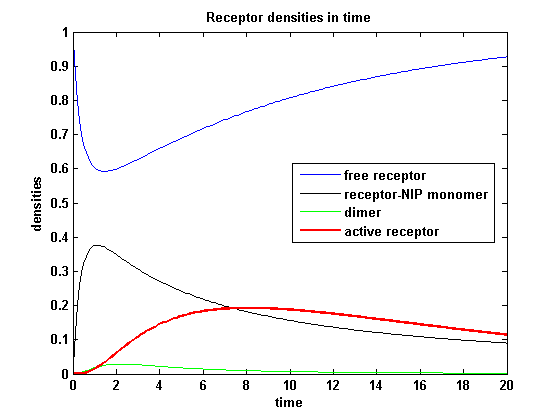
<h1>Modular Synthetic Receptor System model</h1> | <h1>Modular Synthetic Receptor System model</h1> | ||
| - | The dimerization model II is used to form the base of the model for our Modular Synthetic Receptor System. It has two receptors '''R'''<sub>1</sub> and '''R'''<sub>2</sub>. The bivalent ligand are two NIP molecules '''NN''' which are linked to DNA-Origami. Aswell two linked | + | [[media:Freiburg2008_MSRS.m|''matlab m-file'']]<br><br> |
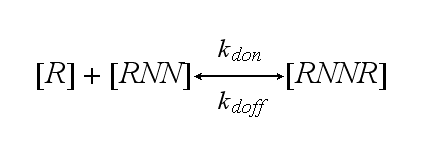
| + | The dimerization model II is used to form the base of the model for our Modular Synthetic Receptor System. It has two receptors '''R'''<sub>1</sub> and '''R'''<sub>2</sub>. The bivalent ligand are two NIP molecules '''NN''' which are linked to DNA-Origami. Aswell two linked fluorescein molecules, or one NIP and fluorescein can serve as the bivalent ligand. Each receptor consists of a extracellular detector domain, which is connected to an intracellular split protein half by a short transmembrane protein. Only when receptor '''R'''<sub>1</sub> and '''R'''<sub>2</sub> dimerize, the intracellular split protein halves complement to become the complete functional protein '''A''' in the form of a β-lactamase or a fluorescent protein. A dimerization of '''R'''<sub>1</sub> and '''R'''<sub>1</sub> or '''R'''<sub>2</sub> and '''R'''<sub>2</sub> can happen to '''R'''<sub>1</sub>'''R'''<sub>1</sub> or '''R'''<sub>2</sub>'''R'''<sub>2</sub>, but does not lead to an active intracellular protein and measurable output. Of course all receptor dimers ('''R'''<sub>1</sub>'''R'''<sub>2</sub>, '''R'''<sub>1</sub>'''R'''<sub>1</sub>, '''R'''<sub>2</sub>'''R'''<sub>2</sub>) dissociate into monomers much easier than a receptor dimer which is active in the form of '''A''', as the connected split protein halves are bound to each other. | ||
<br> | <br> | ||
<h3>Reaction kinetics</h3> | <h3>Reaction kinetics</h3> | ||
| Line 448: | Line 452: | ||
<tr> | <tr> | ||
<td> | <td> | ||
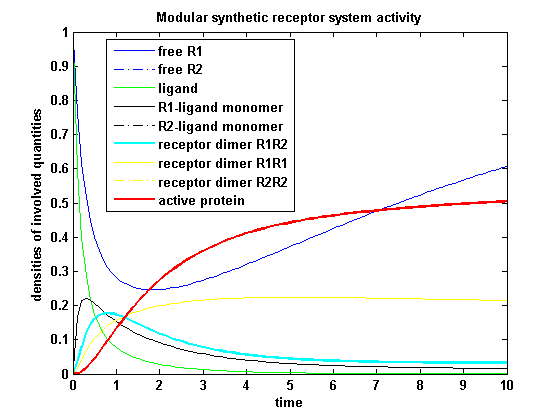
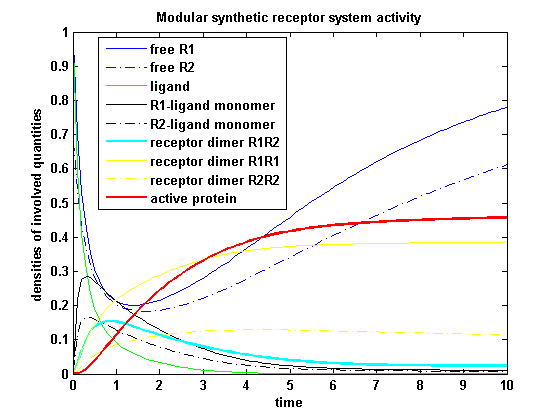
| - | [[image: | + | [[image:Freiburg2008_MSRSp1.png|thumb|left|400px|'''Figure 16:''' Involved receptor densities in time]] |
| - | Assuming equal parameters for R<sub>1</sub> and R<sub>2</sub> leads to equal densities of both; shown through one plot curve for each uniform density (free, monomeric, dimeric density) of R<sub>1</sub> and R<sub>2</sub> | + | Assuming equal parameters for R<sub>1</sub> and R<sub>2</sub> leads to equal densities of both; shown through one plot curve for each uniform density (free receptor density, monomeric density, dimeric density) of R<sub>1</sub> and R<sub>2</sub> |
</td> | </td> | ||
<td>chosen parameters:<br> | <td>chosen parameters:<br> | ||
| Line 459: | Line 463: | ||
K2 = 3; %binding rate 2 | K2 = 3; %binding rate 2 | ||
k2 = 0.1; %dissociating rate 2 | k2 = 0.1; %dissociating rate 2 | ||
| - | K3 = 2; %dimerization rate 1 | + | K3 = 2; %R1R2 dimerization rate 1 |
| - | k3 = 0.1; %dimer dissociating rate 1 | + | k3 = 0.1; %R1R2 dimer dissociating rate 1 |
| - | K4 = 2; %dimerization rate 2 | + | K4 = 2; %R1R2 dimerization rate 2 |
| - | k4 = 0.1; %dimer dissociating | + | k4 = 0.1; %R1R2 dimer dissociating rate 2 |
| - | K5 = 2; % | + | K5 = 2; %R1R1 dimerization rate |
| - | k5 = 0.1; % | + | k5 = 0.1; %R1R1 dimer dissociating rate |
| - | K6 = 2; % | + | K6 = 2; %R2R2 dimerization rate |
| - | k6 = 0.1; % | + | k6 = 0.1; %R2R2 dimer dissociating rate |
Ka = 1; %activation rate | Ka = 1; %activation rate | ||
Kda = 0.05; %deactivation rate | Kda = 0.05; %deactivation rate | ||
| Line 486: | Line 490: | ||
</table> | </table> | ||
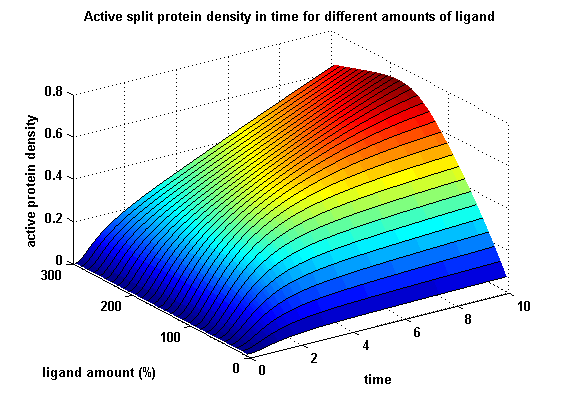
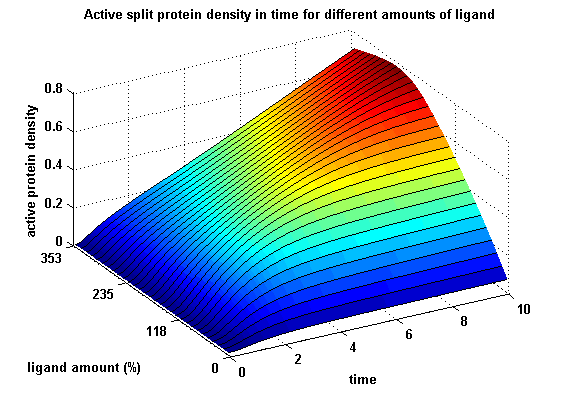
<h3>Active split protein density dependent on input ligand amount</h3> | <h3>Active split protein density dependent on input ligand amount</h3> | ||
| - | In order to gain information about | + | In order to gain information about the split protein activity dependent on the input, the same parameters were used as above except the initial ligand parameter, which was variated. |
<table> | <table> | ||
<tr> | <tr> | ||
<td> | <td> | ||
| - | [[image:Freiburg2008_MSRSdNN.png|thumb|left|600px|'''Figure | + | [[image:Freiburg2008_MSRSdNN.png|thumb|left|600px|'''Figure 17:''' Split protein activity dependent on ligand amount (% of initial free receptor amount)]] |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 496: | Line 500: | ||
<br> | <br> | ||
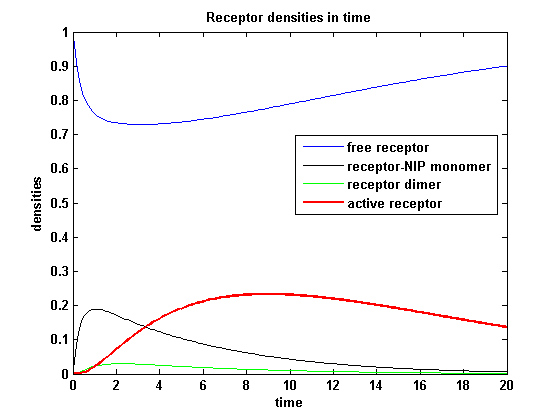
| - | <h3> | + | <h3>Split protein activity with two different kinetic receptors</h3> |
| - | As two different receptors are used, they can differ in the kinetics. Assuming that receptor | + | As two different receptors are used, they can differ in the kinetics. Assuming that receptor R<sub>1</sub> is binding the ligand better, so its binding rate is higher and its dissociating rate is lower than of receptor R<sub>2</sub>. Choosing not only a higher turnover rate s<sub>1</sub> but also a higher dimerization and a lower dimer dissociating rate for receptor R<sub>1</sub> and a lower initial free receptor density for R<sub>2</sub>, gives the following theoretical result: |
<table> | <table> | ||
<tr> | <tr> | ||
<td> | <td> | ||
| - | [[image:Freiburg2008_MSRS_2.png|thumb|left|400px|'''Figure | + | [[image:Freiburg2008_MSRS_2.png|thumb|left|400px|'''Figure 18:''' Involved receptor densities in time with two different kinetic receptors involved]] |
| - | All densities | + | All densities connected to R<sub>2</sub> are shown dashed, except the dimer R<sub>1</sub>R<sub>2</sub>, which becomes the active protein. As stated in the parameters, R<sub>1</sub> is a better binding and dimerizing receptor than R<sub>2</sub> and is available in a higher concentration in the cell in the regarded time course. So all densities (free receptor density, monomeric density, dimeric density) connected to R<sub>1</sub> are higher than the according densities of R<sub>2</sub>. |
</td> | </td> | ||
<td>chosen parameters:<br> | <td>chosen parameters:<br> | ||
| Line 512: | Line 516: | ||
K2 = 3; %binding rate 2 | K2 = 3; %binding rate 2 | ||
k2 = 0.1; %dissociating rate 2 | k2 = 0.1; %dissociating rate 2 | ||
| - | K3 = 2; %dimerization rate 1 | + | K3 = 2; %R1R2 dimerization rate 1 |
| - | k3 = 0.1; %dimer dissociating | + | k3 = 0.1; %R1R2 dimer dissociating rate 1 |
| - | K4 = 2.5; %dimerization rate 2 | + | K4 = 2.5; %R1R2 dimerization rate 2 |
| - | k4 = 0.05; %dimer dissociating rate2 | + | k4 = 0.05; %R1R2 dimer dissociating rate2 |
| - | K5 = 2.5; % | + | K5 = 2.5; %R1R1 dimerization rate |
| - | k5 = 0.05; % | + | k5 = 0.05; %R1R1 dimer dissociating rate |
| - | K6 = 2; % | + | K6 = 2; %R2R2 dimerization rate |
| - | k6 = 0.1; % | + | k6 = 0.1; %R2R2 dimer dissociating rate |
Ka = 1; %activation rate | Ka = 1; %activation rate | ||
Kda = 0.05; %deactivation rate | Kda = 0.05; %deactivation rate | ||
| Line 541: | Line 545: | ||
<tr> | <tr> | ||
<td> | <td> | ||
| - | [[image:Freiburg2008_MSRS_dNN_R1R2d.png|thumb|left|600px|'''Figure | + | [[image:Freiburg2008_MSRS_dNN_R1R2d.png|thumb|left|600px|'''Figure 19:''' Split protein activity dependent on ligand amount (% of initial free receptor amount) with two different kinetic receptors involved]] |
</td> | </td> | ||
</tr> | </tr> | ||
</table> | </table> | ||
| - | <h1>Matlab | + | <br><br> |
| - | <h1>Literature</h1> | + | <h1>Matlab m-files</h1> |
| + | *[[media:Freiburg2008_M1_Basic.m|Basic dimerization model 1]]<br> | ||
| + | *[[media:Freiburg2008_M1_Xten.m|Model 1 with extensions]]<br> | ||
| + | *[[media:Freiburg2008_M1_Corrected.m|Corrected model 1 version]]<br> | ||
| + | *[[media:Freiburg2008_M_2.m|Dimerization Model 2]]<br> | ||
| + | *[[media:Freiburg2008_MSRS.m|Modular Synthetic Receptor System model]]<br> | ||
| + | <br><br> | ||
| + | <h1>[[Image:MO2.jpg|50px|]]Literature</h1> | ||
| + | *Martin F. Bachmann, Michael Salzmann, Annette Oxenius and Pamela S. Ohashi: "Formation of TCR dimers/trimers as a crucial step for T cell activation", Eur. J. Immunol., 1998 | ||
| + | *Martin F. Bachmann and Pamela S. Ohashi: "The role of T-cell receptor dimerization in T-cell activation", Review Immunology Today, 1999 | ||
| + | *João Sousa and Jorge Carneiro: "A mathematical analysis of TCR serial triggering and down-regulation", Eur. J. Immunol., 2000 | ||
| + | *Susana Minguet, Mahima Swamy, Balbino Alarcón, Immanuel F. Luescher and Wolfgang W.A. Schamel: "Full Activation of the T Cell Receptor requires Both Clustering and Conformational Changes at CD3", Immunity, 2006 | ||
}} | }} | ||
Latest revision as of 18:06, 8 March 2009
|
Modeling |
_modeling
IntroductionDimerization of the extracellular receptor domains is an important necessity for the functionality of our Modular Synthetic Receptor System. Presenting the system a stimulus in the form of spatial arranged ligands results in dimerization of the extracellular domains and thus the corresponding intracellular parts such as the split lactamase halves or split fluorescent proteins complement to a measurable output. To analyze the functionality due to dimerization, first two receptor dimerization models (one T cell receptor model and one general receptor model) are introduced and discussed and then a proper model for the Modular Synthetic Receptor System is constructed.
T cell receptor dimerization model Imatlab m-file Extracellular signalingA simplified pathway shows the extracellular sequence of TCR activation. After the NIP binding two complexes come together and form a dimer which then leads to activation of the TCR and further intracellular signaling and T cell activation.
Reaction kinetics
One NIP molecule (N) binds to a TCR (T) with the reaction rate kon or a TCR-NIP complex (TN) dissociates with the reaction rate koff :
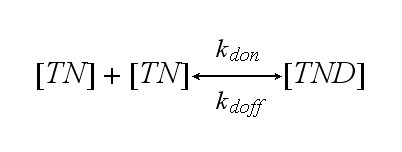
Two TCR-NIP (TN) complexes dimerize to a TCR-NIP dimer (TND) with rate kdon ; the dissociation of a TCR-NIP dimer runs with rate kdoff :
In order to get active TCRs, the TCR-NIP dimer (TND) has to switch into two active TCRs (A) with rate ka :
After activation, the TCR is internalized with rate ki and does not take part anymore in the extracellular signaling :
ODEs derived from the kinetics (Details)In the following equation T represents the free TCR in the T cell membrane where keff is a combination of kdon, kdoff and ka. kI is kon/koff : TCR activity for a set of parametersThe two ODEs above of this first basic model for a set of parameters are solved numerically. They reveal the time course of the TCR activity and the time course of the unbound TCR aswell.
The activity has a maximum a short time after NIP presentation and decreases as the active TCRs are internalized and as the free membrane TCR amount is decreasing. It is remarkable that the response never decreases to complete zero, even for very high internalization rates and in a long time course.
Extensions: Ultrasensitivity and biphasic kineticsUltrasensitivityThe kinetics of the TCR activation can be generalised by substituting the second order kinetic of the ligand N and the receptor T by a parameter h, which then represents the kinetic order of the system. Biphasic kinetics and parameter analysisNot all TCRs on a T cell membrane can be recruited to NIP binding as a cell´s membrane contains several transmembrane proteins whose size can avoid a TCR-NIP formation when they surround a TCR and make the approaching of the NIP to the binding side of the TCR impossible. Considerating this spatial barriers leads to the idea of a TCR which can switch between two states, one binding state and one non-binding state. Hence a introduction of two different pools of TCRs into the model is appropriate. If a TCR is not available for a NIP molecule, thus it is in the non-binding state, it belongs to the so called spare pool, whereby TCRs belonging to the so called interface pool are in the binding state and can be accessed by the NIP molecule. Moreover the spare pool is in dynamical exchange with the interface pool, so non-binding TCRs can become binding TCRs. This exchange is regulated through the parameter λ, a ratio between the spare and the interface pool and φ, the exchange rate constant. S is the spare pool TCR density and T the TCR density of the interface pool. A represents the active TCR density. So the full model I equations are: TCR activity dependent on exchange rate φ and ratio λ :
The x-axis represents the time course of the activity, the y-axis represents both parameters φ ( = y) and λ ( = 2 - y). So each black line in the plot is a time course of the TCR activity for a different φ and λ. The z-axis is the response intensity. With increasing exchange between the interface and spare pool, more TCRs switch to the binding state, hence more TCRs can bind NIP. As a consequence the active TCR density is higher than for a low exchange. Correction termsmatlab m-file
but :
Furthermore can be derived for the NIP density and the free TCR:
After NIP addition the TCR activity rises to a maximum and decreases as active receptors are internalized and the NIP amount is used up. The free receptor density recovers due to permanent expression of new receptors which are built into the membrane.
Receptor dimerization model IImatlab m-file Before the first step the receptors are unbound and diffuse in the cell´s membrane. The DNA-Origami binds to a receptor in the way that only one NIP molecule is connecting to one receptor. In the second step the second NIP molecule binds to the second receptor and both receptors dimerize.
Reaction kineticsThe continuous production of the cell´s receptor R with rate s is:
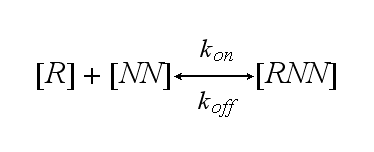
One receptor R binds to one of two NIP molecules NN of the DNA-Origami with rate kon or a receptor-NIP complex RNN dissociates with koff:
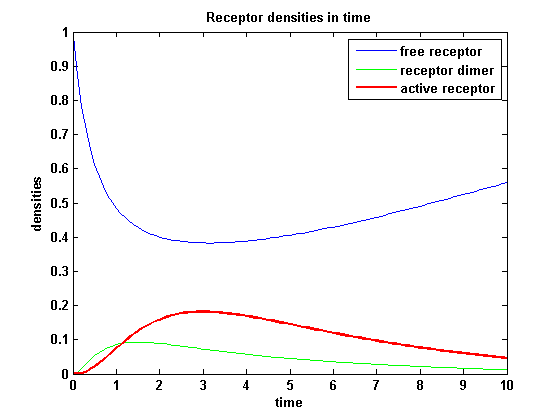
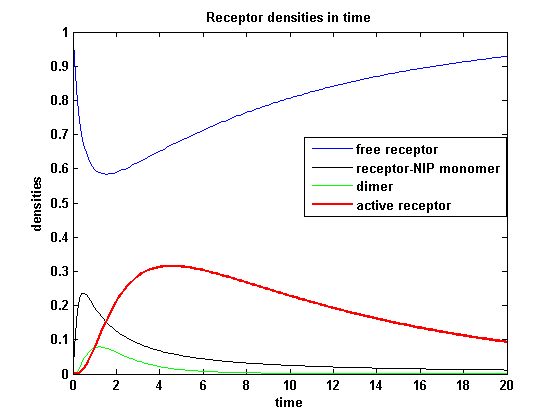
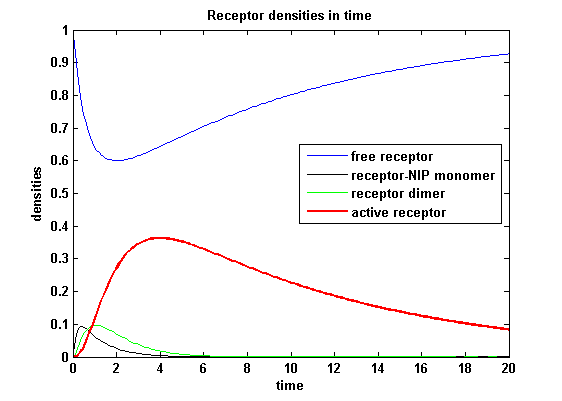
ODE derivationThe ODEs are derived from the reaction kinetics: Extracellular receptor activityFor a set of parameters the ODEs are solved and reveal amongst the other quantities the receptor densities in the time course:
The receptor activity rises after NIP addition, has a maximum and decreases due to the internalization of the active receptor. The dimer density increases fast after addition of NIP and decreases as dimers fade into active receptors.
Discussion of model I and IIFor comparison, the ODEs of the corrected model 1 and model 2 are taken. The receptor NIP binding mechanism differs in the two models. Model I assumes the production of two independent receptor-NIP complexes which can dimerize after formation. In model II, dimerization happens after one of two receptors connects to the bivalent ligand . Setting several conditions unravels the consequences of this differences for the active receptor, the dimer and the receptor recovery. Different binding rates, dimerization rates and ligand amounts are used in the solutions of the model I ODEs and model II ODEs. Receptor densities dependent on binding rate konThe two model ODEs are solved for the mentioned parameters below whereby the binding rate kon was variated.
So the course in time for the free receptor, the monomeric receptor-NIP complex, the dimer and the active receptor is plotted:
kon = 0.2 :
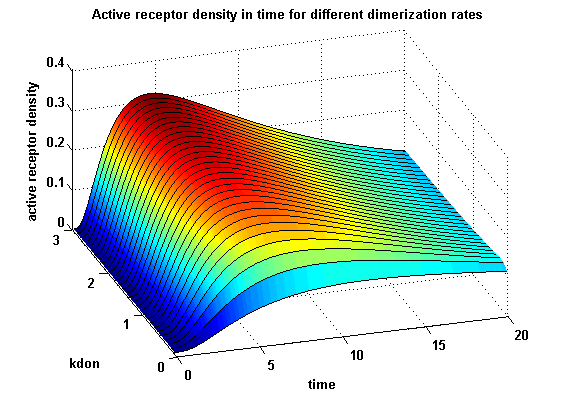
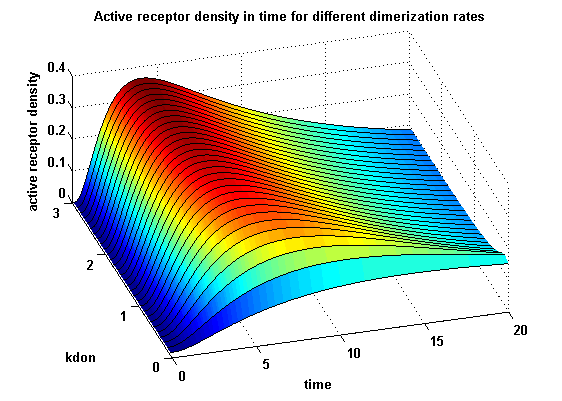
Receptor densities dependent on dimerization rate kdonApplying different kdon leads to the following result:
kdon = 0.3 :
Receptor densities dependent on NIP amountThe use of different amounts of ligand is maybe the most important influence on the receptor activation. As the ligand is the stimulus in order to activate our system, it´s amount will determine strongly the activity of the receptor. So different amounts of NIP are used and a higher internalization rate ki is used aswell.
NIP = 0.1 :
Modular Synthetic Receptor System modelmatlab m-file Reaction kineticsODE derivationThe ODEs can be derived from the reaction kinetics again and are: Involved quantities and split protein activityThe ODEs are solved for a set of parameters and reveal the time courses of the quantities:
Active split protein density dependent on input ligand amountIn order to gain information about the split protein activity dependent on the input, the same parameters were used as above except the initial ligand parameter, which was variated.
Split protein activity with two different kinetic receptorsAs two different receptors are used, they can differ in the kinetics. Assuming that receptor R1 is binding the ligand better, so its binding rate is higher and its dissociating rate is lower than of receptor R2. Choosing not only a higher turnover rate s1 but also a higher dimerization and a lower dimer dissociating rate for receptor R1 and a lower initial free receptor density for R2, gives the following theoretical result:
Matlab m-files
|
 "
"