Team:Freiburg Transfection
From 2008.igem.org
(Difference between revisions)
| Line 6: | Line 6: | ||
'''1) Localisation at the cell membrane'''<br> | '''1) Localisation at the cell membrane'''<br> | ||
To show the localisation of the constructs at the cell membrane transfection of the construct signalpeptide-Lipocalin-transmembraneregion-betaLactamase1-YFP was performed.<br> | To show the localisation of the constructs at the cell membrane transfection of the construct signalpeptide-Lipocalin-transmembraneregion-betaLactamase1-YFP was performed.<br> | ||
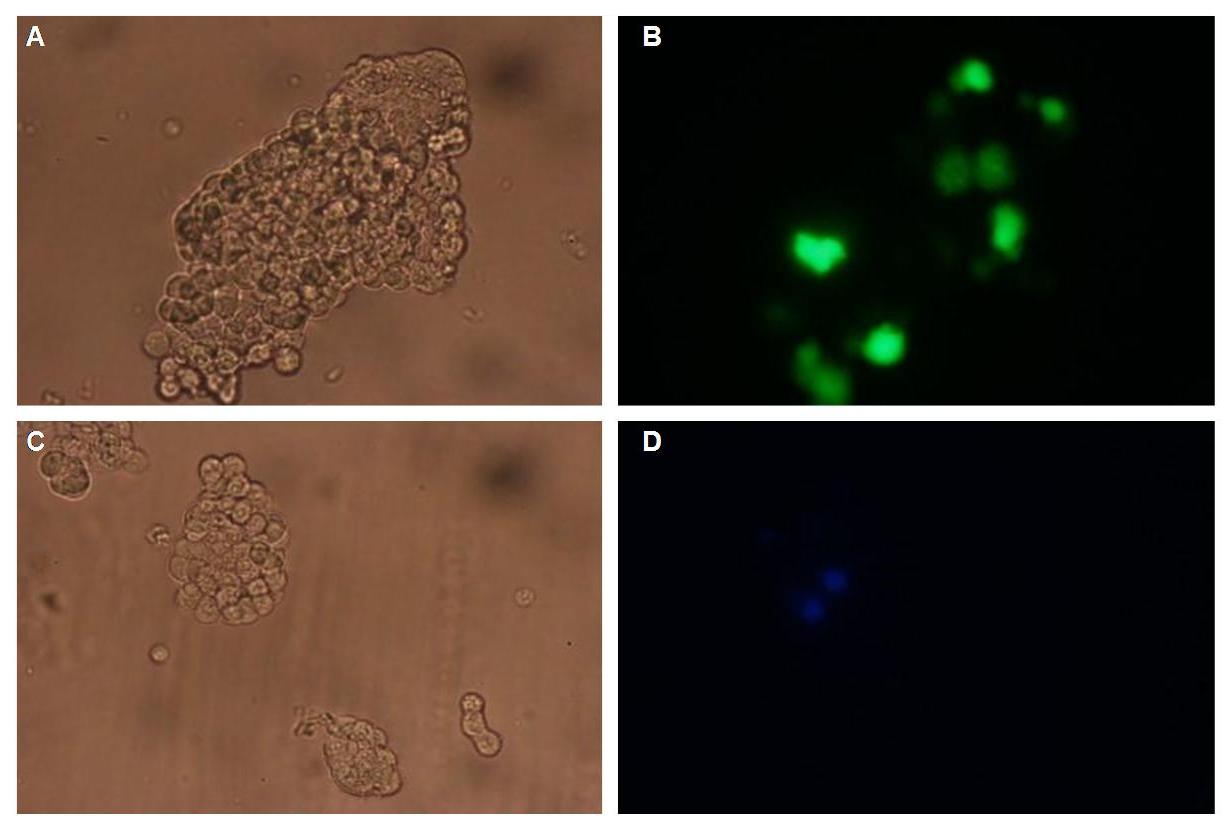
| - | Figure 1_Transfection shows the configuration of the construct. Lipocalin, the fluorescein binding Anticalin, exhibits the extracellular part of the construct. The transmembrane region is appropriate to that of the EGF-receptor erbb1. Split-beta-Lactamase the intracellular part is labeled to the yellow fluorescent protein to detect membrane localization.<br> | + | Figure 1_Transfection shows the configuration of the construct. Lipocalin, the fluorescein binding Anticalin, exhibits the extracellular part of the construct. The transmembrane region is appropriate to that of the EGF-receptor erbb1. Split-beta-Lactamase, the intracellular part is labeled to the yellow fluorescent protein to detect membrane localization.<br> |
<br> | <br> | ||
[[Image:Freiburg2008_Lipo_bla1+YFP.jpg|500px]] | [[Image:Freiburg2008_Lipo_bla1+YFP.jpg|500px]] | ||
| Line 13: | Line 13: | ||
<br> | <br> | ||
<br> | <br> | ||
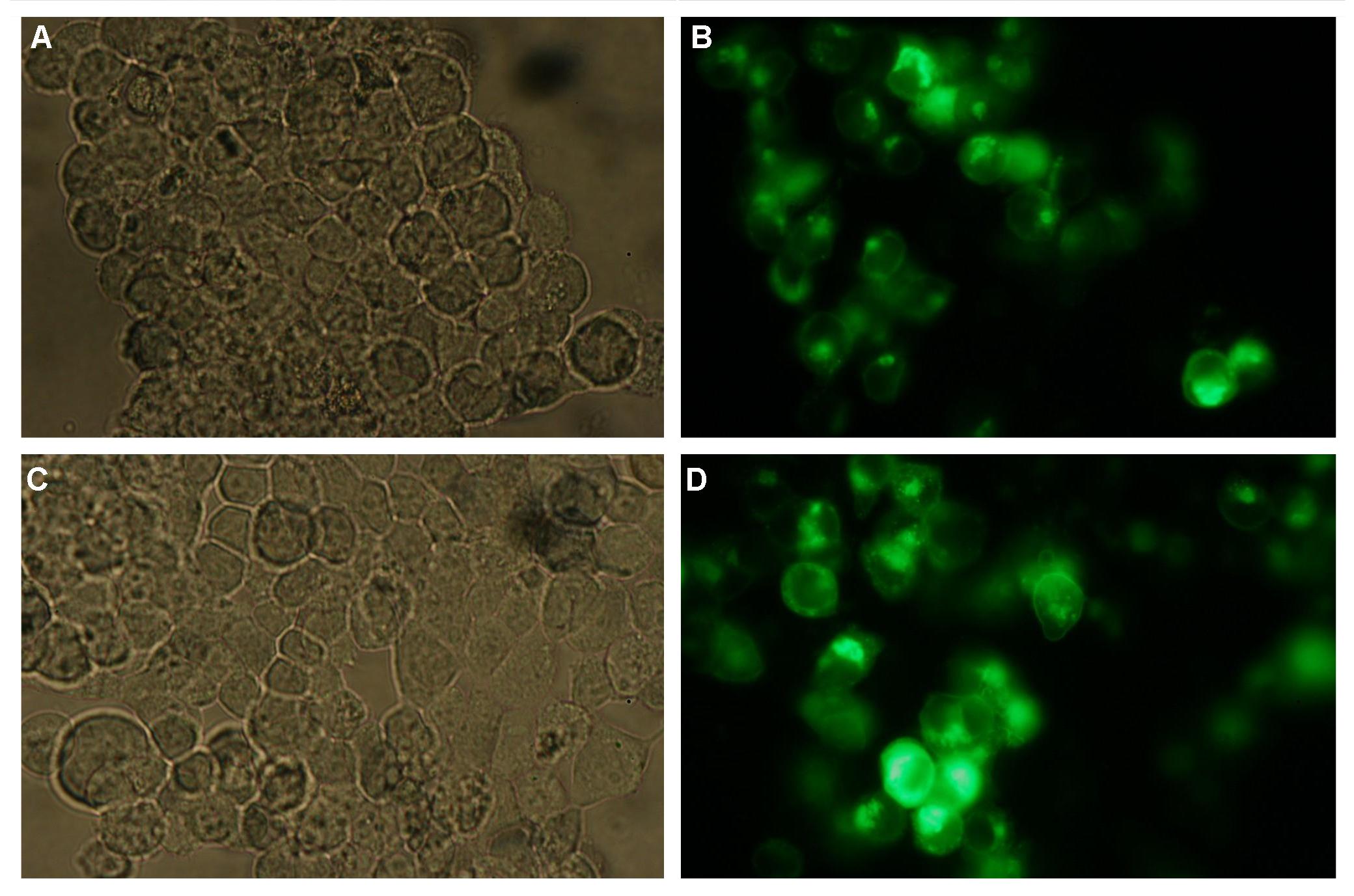
| - | Membranelocalisation of the construct signalpeptide-Lipocalin-transmembraneregion-betaLactamase1-YFP is visible in transfected 293T cells (Figure 2_Transfection). The fluorescence of the cells is most likely restricted to the cellmembrane which confirms the assembly of the construct in the | + | Membranelocalisation of the construct signalpeptide-Lipocalin-transmembraneregion-betaLactamase1-YFP is visible in transfected 293T cells (Figure 2_Transfection). The fluorescence of the cells is most likely restricted to the cellmembrane which confirms the assembly of the construct in the cytoplasmamembrane.<br> |
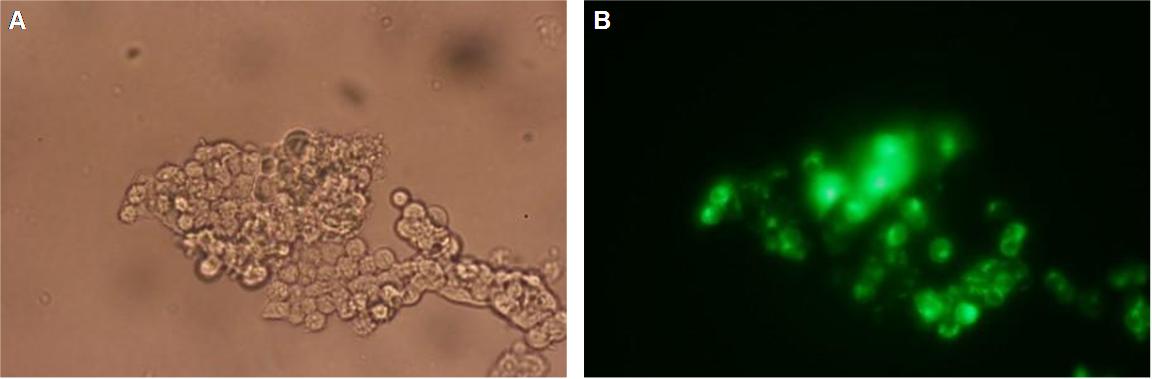
| - | In | + | In comparison, 293T cells transfected with the construct transfectionvector-YFP show a uniformly distributed fluorescence all-over the cell (Figure 3_Tansfection A and B). |
<br> | <br> | ||
[[Image:Freiburg2008_SP_LIPO_GGGS_TM_bla1_YFP_1.jpg|710px]]<br> | [[Image:Freiburg2008_SP_LIPO_GGGS_TM_bla1_YFP_1.jpg|710px]]<br> | ||
Revision as of 14:58, 28 October 2008
 "
"