Team:Hawaii/Ligation of pRL1383a Parts
From 2008.igem.org
(Difference between revisions)
(adding experimetns from 8/11) |
(adding methods and pictures) |
||
| Line 8: | Line 8: | ||
== Methods == | == Methods == | ||
| - | + | ||
| + | <strong> Restriction Digest </strong> | ||
| + | |||
| + | :*Each part is digested with both enzymes. For all cases NEBuffer 2 is used. | ||
| + | |||
| + | :*Reaction Conditions: | ||
| + | ::*5ul Buffer | ||
| + | ::*1ul Enzyme 1 + 1ul Enzyme 2 | ||
| + | ::*0.5 ul BSA | ||
| + | ::* Xul water | ||
| + | ::* Yul insert (1ug if possible) | ||
| + | ::* Zul vector (less than 1ug) | ||
| + | |||
| + | :*Refer to http://openwetware.org/wiki/DNA_ligation for the reasoning behind this. | ||
| + | |||
| + | :*Running Conditions: 2 hours at 37°C. | ||
| + | |||
| + | :*Check the progress of the reaction by running a 0.8% gel. | ||
| + | |||
| + | #<strong>Ligation </strong> | ||
| + | |||
| + | {| border="1" | ||
| + | |+ ''' Ligation''' | ||
| + | !width="100"|Name | ||
| + | |- | ||
| + | |rep(80ng)+B0030(25ng) | ||
| + | |- | ||
| + | |oriV(20ng)+pSB1A2(50ng) | ||
| + | |- | ||
| + | |aadA (BB) | ||
| + | |} | ||
| + | |||
| + | #Transformation | ||
| + | |||
| + | #Verification with Colony PCR | ||
| + | |||
| + | == Results == | ||
| + | |||
| + | ===8/11=== | ||
| + | |||
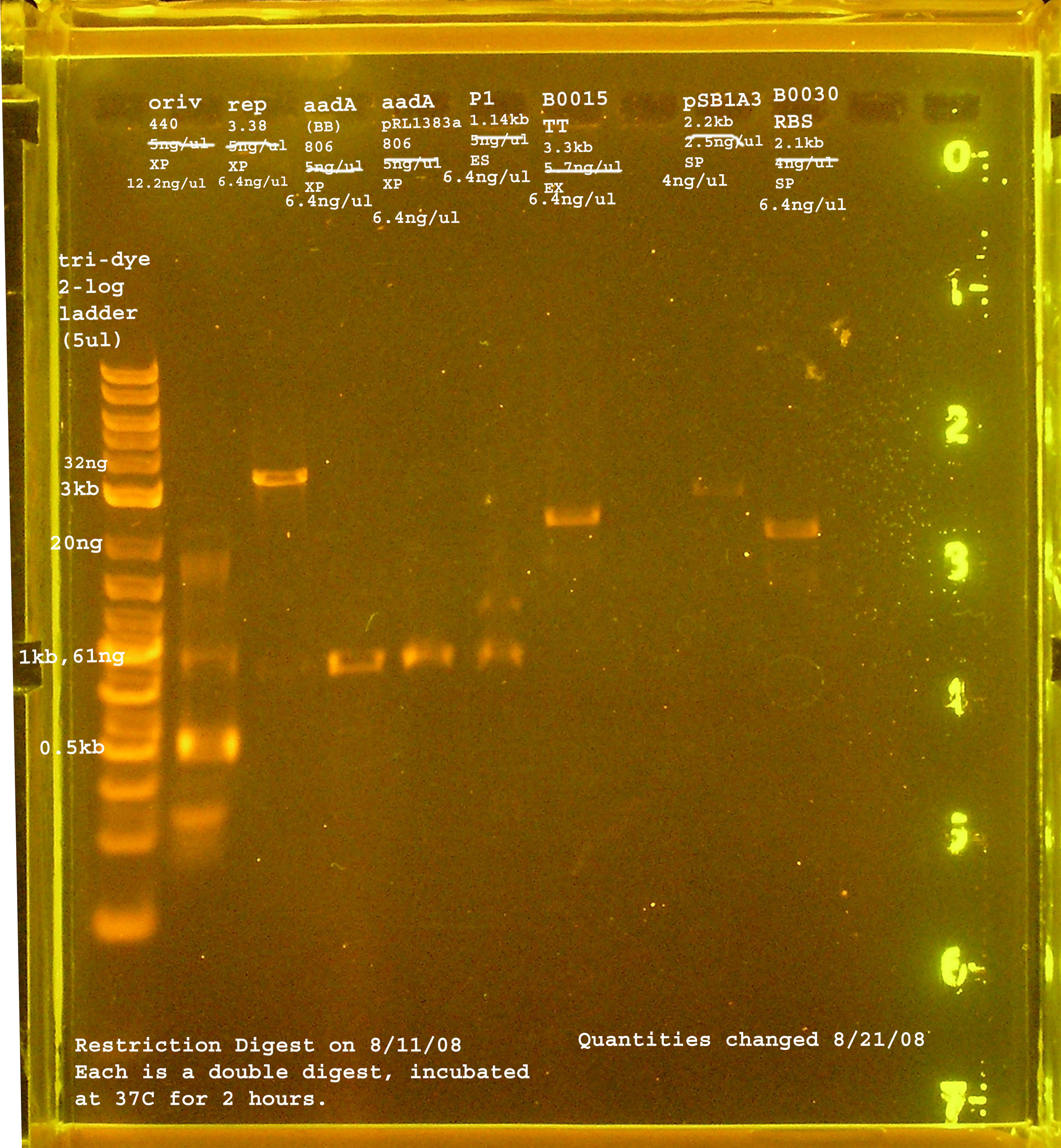
| + | [[Image:re_digest_8_11_08.jpg|right|thumb|300px|Restriction digest from 8/11/08.]] | ||
{| border="1" | {| border="1" | ||
| Line 78: | Line 118: | ||
|n/a | |n/a | ||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
* <br> | * <br> | ||
Revision as of 02:45, 14 August 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Contents |
Ligation of Parts
- The BioBrick parts of pRL1383a are to be ligated in a series of experiments.
Methods
Restriction Digest
- Each part is digested with both enzymes. For all cases NEBuffer 2 is used.
- Reaction Conditions:
- 5ul Buffer
- 1ul Enzyme 1 + 1ul Enzyme 2
- 0.5 ul BSA
- Xul water
- Yul insert (1ug if possible)
- Zul vector (less than 1ug)
- Refer to http://openwetware.org/wiki/DNA_ligation for the reasoning behind this.
- Running Conditions: 2 hours at 37°C.
- Check the progress of the reaction by running a 0.8% gel.
- Ligation
| Name |
|---|
| rep(80ng)+B0030(25ng) |
| oriV(20ng)+pSB1A2(50ng) |
| aadA (BB) |
- Transformation
- Verification with Colony PCR
Results
8/11
| Name | size | enzyme | quantity | date of digest |
|---|---|---|---|---|
| rep | 3.3kb | XbaI & PstI | 5ng/ul | 8/11/08 |
| oriV | 415bp | XbaI & PstI | 5ng/ul | 8/11/08 |
| aadA (pRL1383a) | 806bp | XbaI & PstI | 5ng/ul | 8/11/08 |
| aadA (BB) | 806bp | XbaI & PstI | 5ng/ul | 8/11/08 |
| oriT | ~125bp | XbaI & PstI | n/a | 8/27/08 |
| P1 lytic Region | 1.3kb | EcoRI & SpeI | 5ng/ul | 8/11/08 |
| BBa_B0030 | 2094bp | SpeI & PstI | 4ng/ul | 8/11/08 |
| BBa_B0015 | 3318bp | EcoRI & XbaI | 5.7ng/ul | 8/11/08 |
| pSB1A2 | 2094bp | SpeI & PstI | 5.5ng/ul | 8/11/08 |
| BBa_I14032 | 4462bp | n/a | n/a |
Discussion
- What was learned and how to do future experiments differently.
 "
"