Team:Hawaii/Ligation of pRL1383a Parts
From 2008.igem.org
Revision as of 03:07, 16 August 2008 by MargaretRuzicka (Talk | contribs)
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Contents |
Ligation of Parts
- The BioBrick parts of pRL1383a are to be ligated in a series of experiments.
Methods
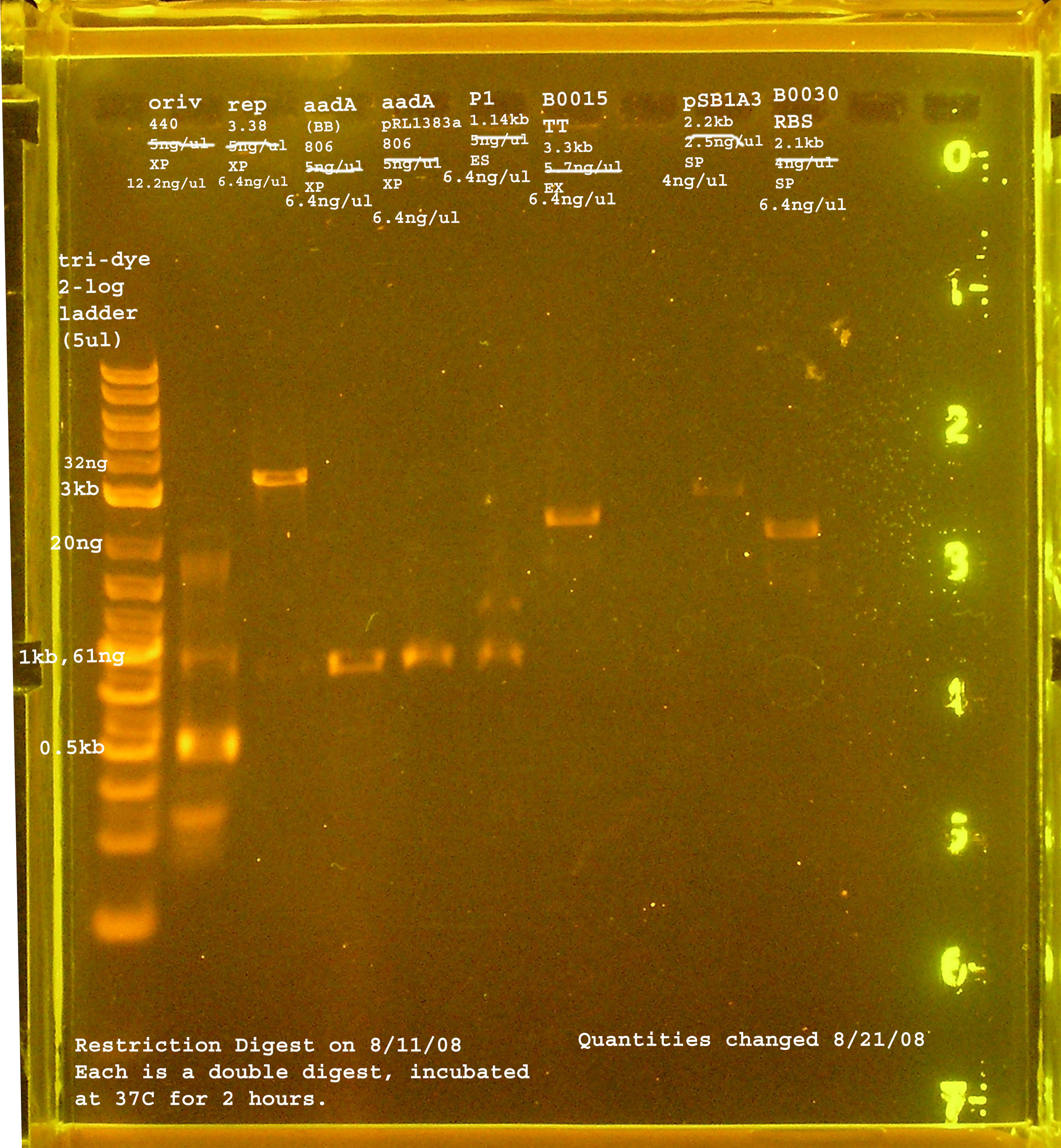
Restriction Digest
- Each part is digested with both enzymes. For all cases NEBuffer 2 is used.
- Reaction Conditions:
- 5ul Buffer
- 1ul Enzyme 1 + 1ul Enzyme 2
- 0.5 ul BSA
- Xul water
- Yul insert (1ug if possible)
- Zul vector (less than 1ug)
- Running Conditions: 2 hours at 37°C.
- Check the progress of the reaction by running a 0.8% gel.
Ligation
- Refer to http://openwetware.org/wiki/DNA_ligation for the reasoning behind this.
Transformation
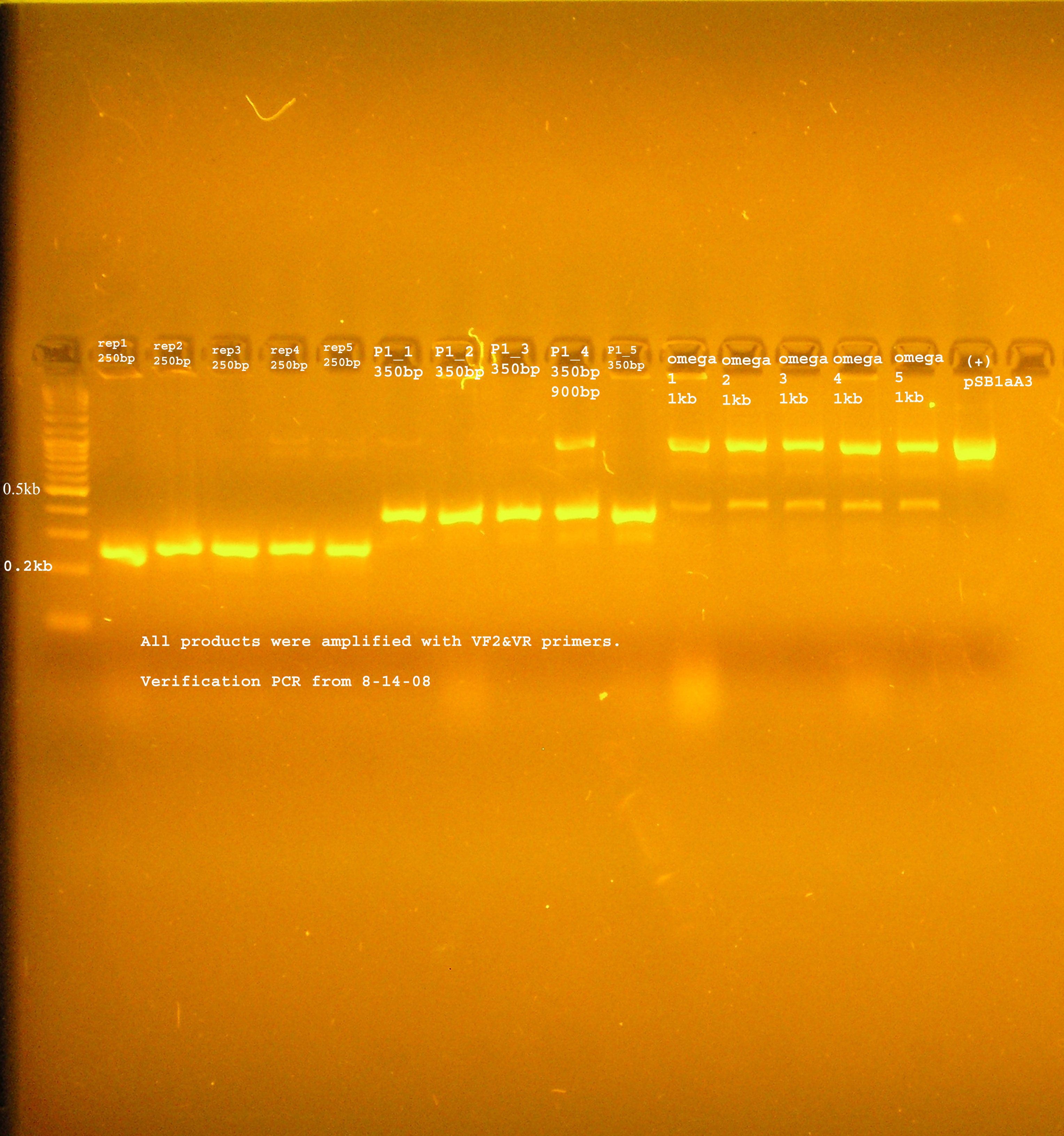
Verification with Colony PCR
Results
7/27
- A few re-digests and ligations were performed today: oriT + pSB1A3, oriV + pSB1A3, mob + B0024, rep + R0010, aadA(pRL1383a+R0010). Afterwards, the ligation product was transformed to DH5-alpha. Only OriT transformed. I did not check the progress of this experiment with a gel, so I am not sure where this went wrong.
- Next time, a gel will be run after every step.
| Name | size | enzyme | quantity |
|---|---|---|---|
| oriT | ~125bp | XbaI & PstI | n/a |
8/11
| Name | size | enzyme | quantity |
|---|---|---|---|
| rep | 3.3kb | XbaI & PstI | 5ng/ul |
| oriV | 415bp | XbaI & PstI | 5ng/ul |
| aadA (pRL1383a) | 806bp | XbaI & PstI | 5ng/ul |
| aadA (BB) | 806bp | XbaI & PstI | 5ng/ul |
| P1 lytic Region | 1.3kb | EcoRI & SpeI | 5ng/ul |
| [http://partsregistry.org/Part:BBa_B0030 BBa_B0030] | 2094bp | SpeI & PstI | 4ng/ul |
| [http://partsregistry.org/Part:BBa_B0015 BBa_B0015] | 3318bp | EcoRI & XbaI | 5.7ng/ul |
| [http://partsregistry.org/Part:pSB1A2 pSB1A2] | 2094bp | SpeI & PstI | 5.5ng/ul |
| Name | strain, antibiotics | colonies? after next day??? | PCR verification |
|---|---|---|---|
| rep(20ng)+B0030(16ng) | DH5-alpha & Amp100 | lawn | |
| oriV(20ng)+pSB1A2(50ng) | DH5-alpha & Amp100 | ||
| aadA (BB)+ B0030 | DH5-alpha & Amp100 | ||
| aadA(pRL1383a)+B0030 | DH5-alpha & Amp100 | ||
| P1 lytic + B0015 | DH5-alpha & Kan50 |
8/14
Discussion
- What was learned and how to do future experiments differently.
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"