Team:Hawaii/Notebook/2008-08-17
From 2008.igem.org
(Difference between revisions)
(→Verification of transformants) |
(→Verification of transformants) |
||
| (4 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
===Verification of transformants=== | ===Verification of transformants=== | ||
:<strong> Grace</strong> | :<strong> Grace</strong> | ||
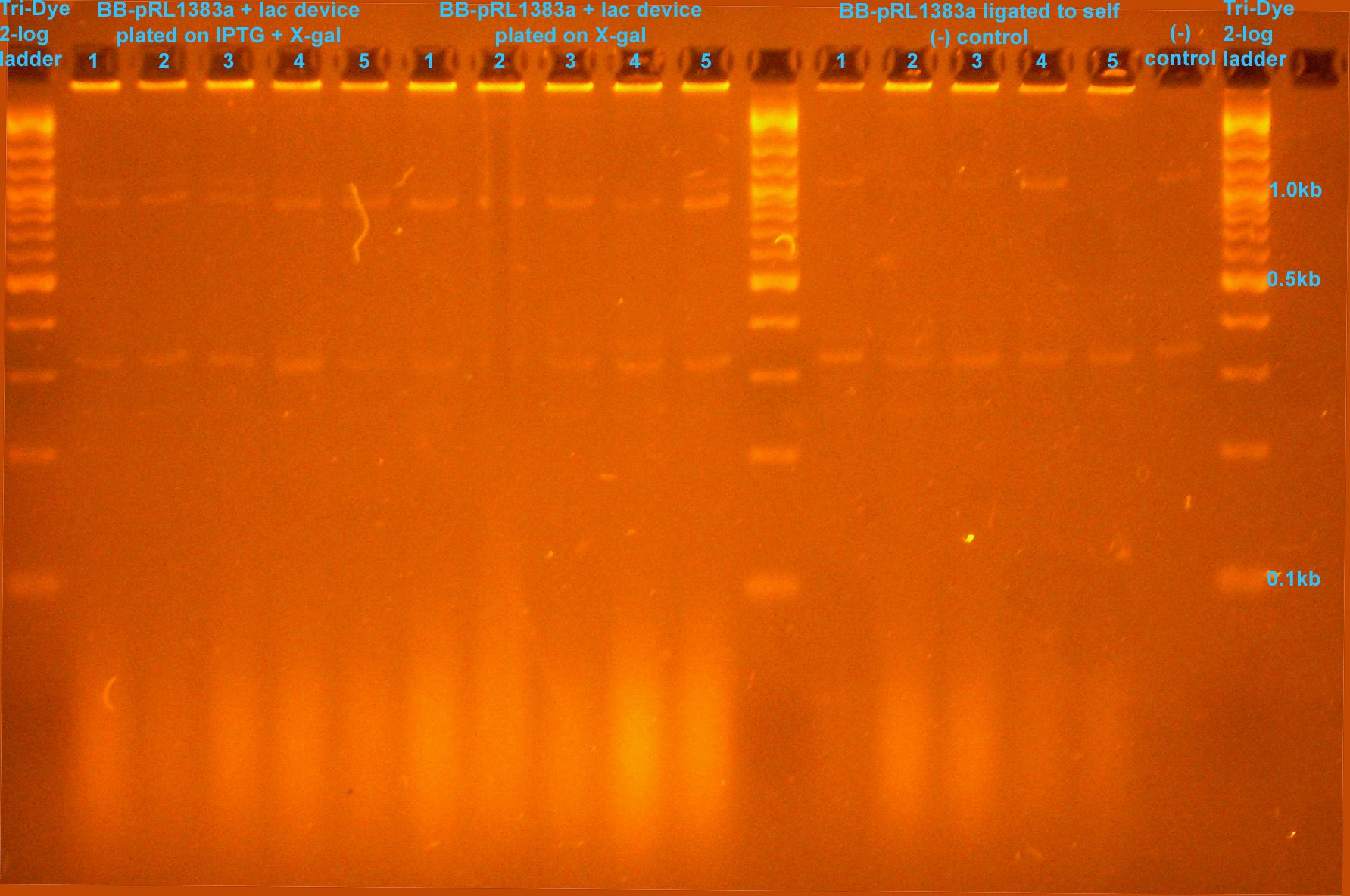
| - | [[Image:081708colonyPCR.jpg|right|thumb| | + | [[Image:081708colonyPCR.jpg|right|thumb|350px|EtBr stained 2.5% agarose gel ran at 95V for 1 .5 hours. Five microliters of PCR reaction were loaded into each well.]] |
{| class=wikitable border=1 align=center | {| class=wikitable border=1 align=center | ||
! DNA | ! DNA | ||
| + | ! Plate | ||
! Total colony forming units | ! Total colony forming units | ||
! Blue colonies | ! Blue colonies | ||
|- | |- | ||
| - | | align=center|BB-pRL1383a + J33207 | + | | align=center|BB-pRL1383a <br>+ J33207 |
| - | | align=center| | + | | align=center|LB + sp<sub>100</sub> + sm<sub>50</sub> +<br> IPTG (1mM) + X-gal (2mM) |
| - | |align=center| | + | |align=center|105 |
| + | |align=center|0 | ||
|- | |- | ||
| - | | align=center|BB-pRL1383a + J33207 | + | | align=center|BB-pRL1383a <br>+ J33207 |
| - | |align=center| | + | |align=center|LB + sp<sub>100</sub> + X-gal (2mM) |
| - | |align=center| | + | |align=center|44 |
| + | |align=center|0 | ||
|- | |- | ||
| - | |align=center| BB-pRL1383a (negative control) | + | |align=center| BB-pRL1383a <br>(negative control) |
| - | |align=center| | + | |align=center|LB + sp<sub>100</sub> + sm<sub>50</sub> +<br> IPTG (1mM) + X-gal (2mM) |
| - | |align=center| | + | |align=center|44 |
| + | |align=center|0 | ||
|- | |- | ||
| - | | align=center|no DNA (negative control) | + | | align=center|no DNA <br>(negative control) |
| - | |align=center| | + | |align=center|LB + sp<sub>100</sub> + sm<sub>50</sub> +<br> IPTG (1mM) + X-gal (2mM) |
| - | |align=center| | + | |align=center|0 |
| + | |align=center|0 | ||
|} | |} | ||
| + | :* X-gal did not work? | ||
:* Colony PCR to verify insert | :* Colony PCR to verify insert | ||
| + | ::* ~320bp band is due to pRL1383a plasmid | ||
| + | ::* BB-pRL1383a + J33207 bands are smaller than (-) control bands, potential success in replacing GFP w/ lac device | ||
| + | :::* (-) bands due to contamination | ||
| + | :* Restreaked colonies to purify | ||
| + | :* Grew up colonies in TB+sp<sub>100</sub> liquid media for plasmid prep on Tuesday | ||
= Discussion = | = Discussion = | ||
Latest revision as of 05:32, 18 August 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Things we did today
Wetlab work
Verification of transformants
- Grace
| DNA | Plate | Total colony forming units | Blue colonies |
|---|---|---|---|
| BB-pRL1383a + J33207 | LB + sp100 + sm50 + IPTG (1mM) + X-gal (2mM) | 105 | 0 |
| BB-pRL1383a + J33207 | LB + sp100 + X-gal (2mM) | 44 | 0 |
| BB-pRL1383a (negative control) | LB + sp100 + sm50 + IPTG (1mM) + X-gal (2mM) | 44 | 0 |
| no DNA (negative control) | LB + sp100 + sm50 + IPTG (1mM) + X-gal (2mM) | 0 | 0 |
- X-gal did not work?
- Colony PCR to verify insert
- ~320bp band is due to pRL1383a plasmid
- BB-pRL1383a + J33207 bands are smaller than (-) control bands, potential success in replacing GFP w/ lac device
- (-) bands due to contamination
- Restreaked colonies to purify
- Grew up colonies in TB+sp100 liquid media for plasmid prep on Tuesday
Discussion
Quote of the Day
History is the only laboratory we have in which to test the consequences of thought. - Étienne Gilson
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"