Team:Hawaii/Notebook/2008-10-13
From 2008.igem.org
(Difference between revisions)
(→Verification of transformants) |
(→Triparental Conjugation) |
||
| (4 intermediate revisions not shown) | |||
| Line 8: | Line 8: | ||
:* Colony PCR of restreak plates | :* Colony PCR of restreak plates | ||
::* Bands ~600bp. Plasmid did not cut. | ::* Bands ~600bp. Plasmid did not cut. | ||
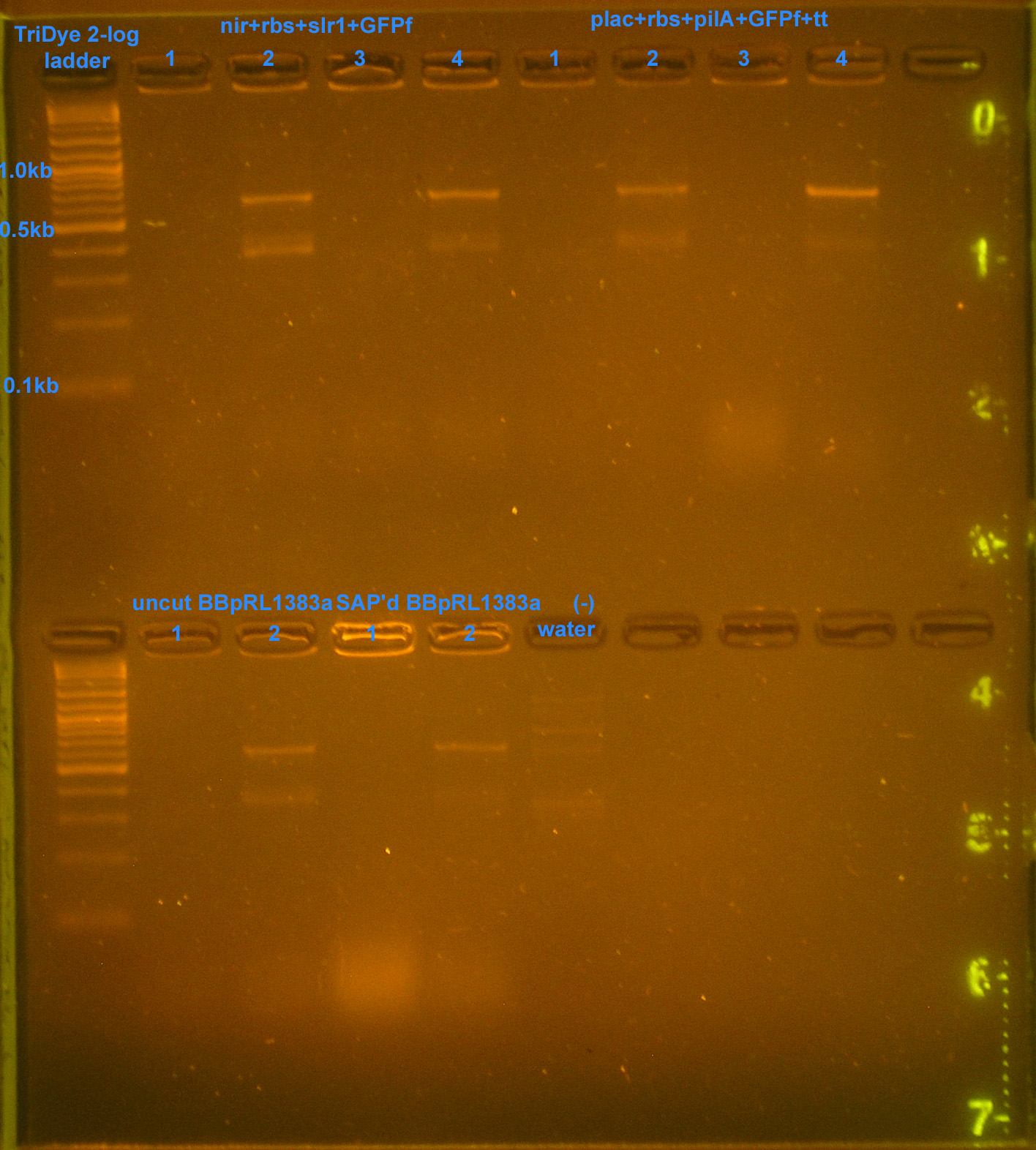
| - | :* | + | [[Image:101308REdigest.jpg|right|thumb|250px|EtBr stained 2% agarose gel ran at 60V for 2 hours. Thirty microliters of RE digested plasmid were loaded into each lane.]] |
| + | :<strong>Krystle</strong> | ||
| + | :* RE digest of plasmid w/ EcoRI and PstI | ||
| + | :* RE digest of prpgt, rgt1 plasmids with EcoRI and PstI | ||
| + | :* Ran RE digests on gel | ||
| + | :* Bands did not correspond to what was supposedly loaded | ||
| + | |||
| + | :<strong>Grace</strong> | ||
| + | :* PCR of prpgt #5, 7, 11, nrsg #6, rgt #1 | ||
| + | :* Overnight RE digest of devices with EcoRI and PstI and rgt#1 with XbaI and PstI | ||
===Triparental Conjugation=== | ===Triparental Conjugation=== | ||
| + | |||
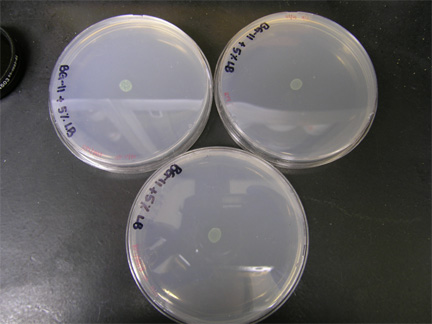
| + | [[Image:101308triparents.jpg|right|thumb|200px|Triparental conjugation plates. RP1, BB-pRL harboring ''E. coli'' and ''Synechocystis'' sp. PCC 6803 plated on 5% LB + BG-11 and incubated for 60 hours at 37C with 2% CO<sub>2</sub>]] | ||
:<strong>Grace</strong> | :<strong>Grace</strong> | ||
| - | |||
:* Restreaked 5%LB+BG-11 plates onto BG-11+sp<sub>2.5</sub>+sm<sub>2.5</sub> plates for isolation of cyanos | :* Restreaked 5%LB+BG-11 plates onto BG-11+sp<sub>2.5</sub>+sm<sub>2.5</sub> plates for isolation of cyanos | ||
:* Inoculated BG-11+sp<sub>2.5</sub>+sm<sub>2.5</sub> liquid media with conjugated ''E. coli''/cyanos | :* Inoculated BG-11+sp<sub>2.5</sub>+sm<sub>2.5</sub> liquid media with conjugated ''E. coli''/cyanos | ||
:* Incubated at 37C with 2% CO<sub>2</sub> for 11 days. Liquid cultures were shaken at 100 rpm also. | :* Incubated at 37C with 2% CO<sub>2</sub> for 11 days. Liquid cultures were shaken at 100 rpm also. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
= Discussion = | = Discussion = | ||
Latest revision as of 20:27, 18 October 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Things we did today
Wetlab work
Verification of transformants
- Grace
- Colony PCR of restreak plates
- Bands ~600bp. Plasmid did not cut.
File:101308REdigest.jpg
EtBr stained 2% agarose gel ran at 60V for 2 hours. Thirty microliters of RE digested plasmid were loaded into each lane.
- Krystle
- RE digest of plasmid w/ EcoRI and PstI
- RE digest of prpgt, rgt1 plasmids with EcoRI and PstI
- Ran RE digests on gel
- Bands did not correspond to what was supposedly loaded
- Grace
- PCR of prpgt #5, 7, 11, nrsg #6, rgt #1
- Overnight RE digest of devices with EcoRI and PstI and rgt#1 with XbaI and PstI
Triparental Conjugation
- Grace
- Restreaked 5%LB+BG-11 plates onto BG-11+sp2.5+sm2.5 plates for isolation of cyanos
- Inoculated BG-11+sp2.5+sm2.5 liquid media with conjugated E. coli/cyanos
- Incubated at 37C with 2% CO2 for 11 days. Liquid cultures were shaken at 100 rpm also.
Discussion
Quote of the Day
History is the only laboratory we have in which to test the consequences of thought. - Étienne Gilson
 "
"