Team:Hawaii/Notebook/2008-10-21
From 2008.igem.org
(Difference between revisions)
(→Antibiotic test for BB-pRL1383a) |
(→Wetlab work) |
||
| (7 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
== Wetlab work == | == Wetlab work == | ||
===Insertion of lac-GFP device into BB-pRL1383a=== | ===Insertion of lac-GFP device into BB-pRL1383a=== | ||
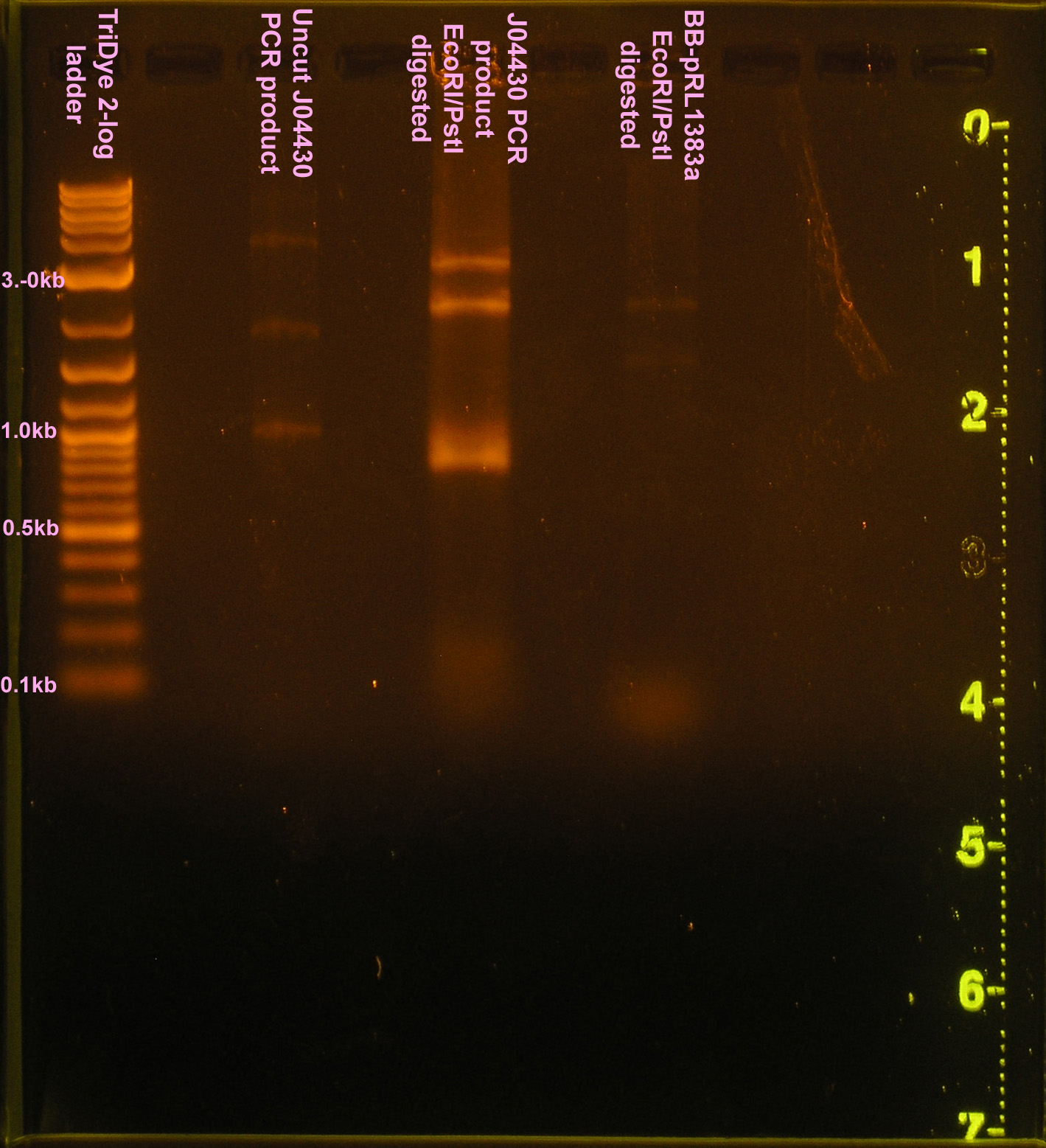
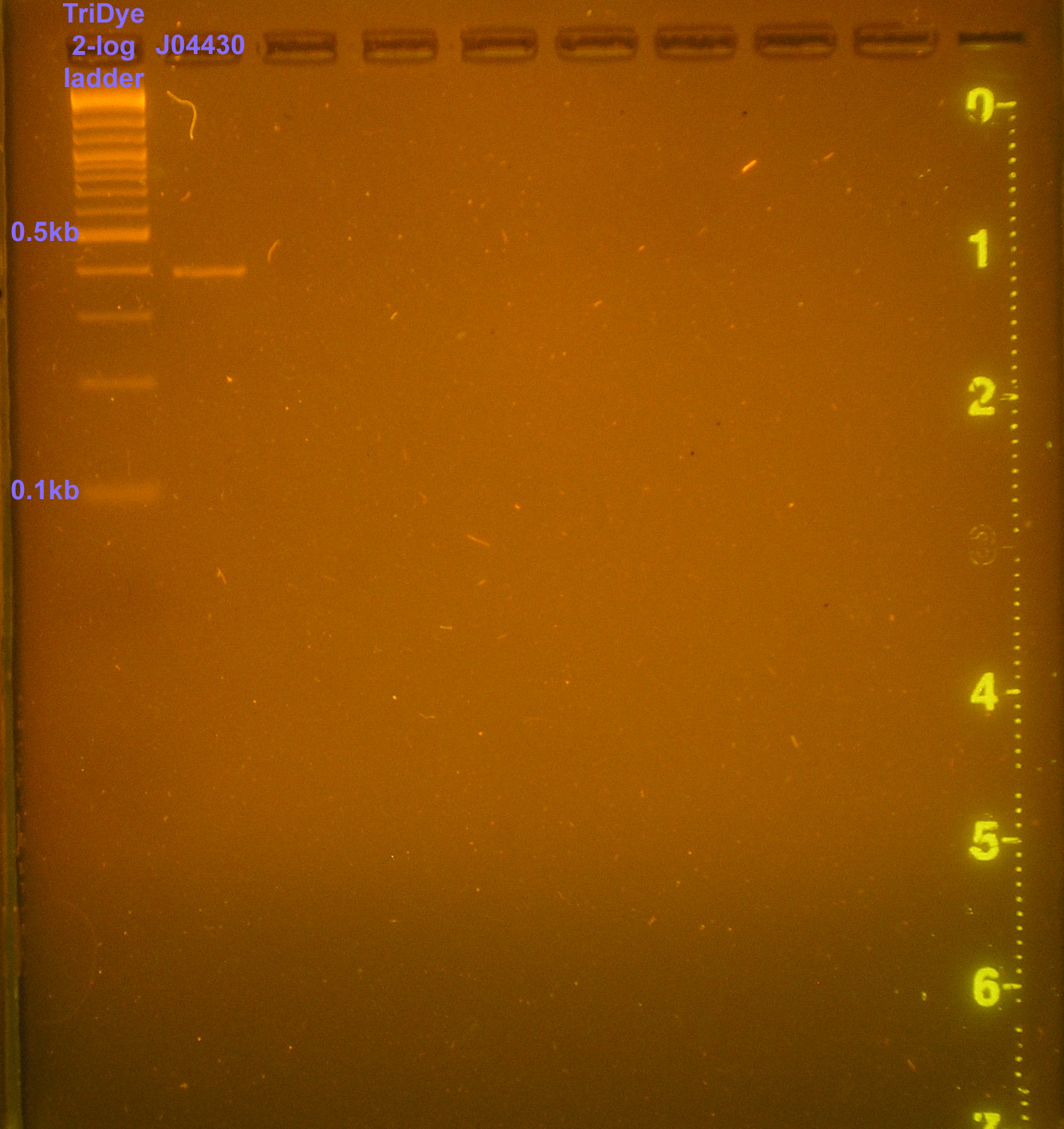
| + | [[Image:102108REdigests.png|right|thumb|150px|EtBr stained 0.8% agarose gel ran at 60V for 90 minutes. Thirty microliters of the RE digest reaction were loaded into each well.]][[Image:102108J04430.png|right|thumb|150px|EtBr stained 0.8% agarose gel ran at 60V for 90 minutes. Ten microliters of PCR product were loaded.]] | ||
| + | |||
:<strong> Grace</strong> | :<strong> Grace</strong> | ||
| - | + | ||
:*Ran RE digests on gel | :*Ran RE digests on gel | ||
| - | ::* | + | ::*Bands were all incorrect size |
| - | :* | + | :* Reinoculated TB+sp<sub>100</sub> for plasmid prep tomorrow |
| - | :* | + | :* PCR of J04430 from filter paper to verify part; used H/B primers |
| - | + | ||
| - | ===Antibiotic test for BB-pRL1383a=== | + | ===[[Team:Hawaii/Antibiotic test for BB-pRL1383a]]=== |
:<strong> Grace</strong> | :<strong> Grace</strong> | ||
| - | :* Made LB+sp<sub>variable</sub> plates with | + | :* Made LB+sp<sub>variable</sub> plates with 50 μl 20X X-gal |
| - | :* Transformed 100 μl DH5α with 2 & | + | :* Transformed 100 μl DH5α with 2 μl BB-pRL1383a (4 ng) |
| - | :* Plated 50 & | + | :* Plated 50 μl cells + SOB on each LB+sp plate; plated 20 μl untransformed cells + SOB on each control plate |
:* Incubated at 37C for 2 days | :* Incubated at 37C for 2 days | ||
| + | |||
| + | ===Construction of GFP secretion device=== | ||
| + | :<strong>Krystle</strong> | ||
| + | :* Gel purified overnight restriction digested nir+rbs | ||
| + | ::* Loaded all of restriction digest into a 2% gel, which appeared blank after running for 1.5 hours | ||
| + | :* Re-PCR nir+rbs and slr+gfpf from plasmid prep | ||
| + | :* Restriction digest | ||
| + | ::*nir+rbs with EcoRI and SpeI | ||
| + | ::*slr+gfpf with XbaI and PstI | ||
= Discussion = | = Discussion = | ||
Latest revision as of 01:05, 30 October 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Things we did today
Wetlab work
Insertion of lac-GFP device into BB-pRL1383a
- Grace
- Ran RE digests on gel
- Bands were all incorrect size
- Reinoculated TB+sp100 for plasmid prep tomorrow
- PCR of J04430 from filter paper to verify part; used H/B primers
Team:Hawaii/Antibiotic test for BB-pRL1383a
- Grace
- Made LB+spvariable plates with 50 μl 20X X-gal
- Transformed 100 μl DH5α with 2 μl BB-pRL1383a (4 ng)
- Plated 50 μl cells + SOB on each LB+sp plate; plated 20 μl untransformed cells + SOB on each control plate
- Incubated at 37C for 2 days
Construction of GFP secretion device
- Krystle
- Gel purified overnight restriction digested nir+rbs
- Loaded all of restriction digest into a 2% gel, which appeared blank after running for 1.5 hours
- Re-PCR nir+rbs and slr+gfpf from plasmid prep
- Restriction digest
- nir+rbs with EcoRI and SpeI
- slr+gfpf with XbaI and PstI
Discussion
Quote of the Day
History is the only laboratory we have in which to test the consequences of thought. - Étienne Gilson
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"